Ten years of glory is unusual: anti-cancer drug inventory
Ten years of glory is unusual: anti-cancer drug inventory February 03, 2015 Source: Lilac Garden Since its first clinical cancer progress report was released in 2005, ASCO has witnessed solid and steady progress in the field of oncology for 10 years. Over the past 10 years, more than 60 anti-cancer drugs have been approved by the FDA (Figure 1). With the deepening of understanding of cancer biology, scientists have developed a series of new molecular targeted drugs, and their introduction has changed thousands. The current status of tens of thousands of cancer patients who are difficult to treat. Such novel drugs can target specific molecules or clusters of molecules necessary for tumor cell growth, survival or proliferation. Ten years ago, the National Institutes of Health launched the TCGA program, which became the earliest and most extensive of such projects. To date, the TCGA research network has mapped complete molecular maps of 10 different cancer types. Today, TCGA and other high-throughput sequencing projects continue to explore valuable information that will help improve patient outcomes through a range of pathways. It is possible to select the most appropriate treatment, and new cancer-driven genetic abnormalities have been identified. These genes may become targets for new drugs. After decades of steady development, the field of antibody immunotherapy has finally ushered in a long-awaited major success in the treatment of advanced melanoma, followed by a series of other cancer types, including lung cancer. Common types have also progressed. The survival of patients who had previously lacked effective treatments was significantly prolonged after treatment with new therapies. A recent long-term study suggests that antibody immunotherapy still has an effect on tumor growth after many years of treatment. Another immunotherapy is dedicated to recombining its own immune cells to attack tumor cells, which also excels for specific blood tumors as well as a range of solid tumors. The first cancer vaccine has also been available in the past decade (cervical cancer Gardasil vaccine). Experiments to explore other types of cancer vaccines are also underway. Finally, large-scale screening studies have brought new and important evidence that screening practices can be advanced for some common cancers such as lung, breast and prostate cancer. Targeted therapy develops rapidly In the last decade, we have seen a steady and rapid increase in new targeted therapies approved by the FDA, far exceeding the rate of development of new chemotherapeutic drugs (Figure 2). Approximately 40 new targeted drugs were approved during this period, many of which changed the traditional treatment modality and greatly improved the prognosis of many cancer patients. New tumor starvation therapy First, an anti-angiogenic inhibitor, a class of drugs designed to reduce tumor angiogenesis, has been introduced and has become a successful treatment for many advanced and aggressive cancers. The first such drug approved by the FDA is bevacizumab, which was approved for advanced colorectal cancer in 2004 and has since been used in certain lung, kidney, ovarian, and brain tumors. Subsequently, other angiogenesis inhibitors such as axitinib, cabozantinib, pazopanib, regorafenib, sorafenib, sunitinib, vandetanib and abecept were successively Approved for the treatment of advanced kidney cancer, pancreatic cancer, colorectal cancer, thyroid cancer, and gastrointestinal stromal tumors and sarcomas. EGFR inhibitors: targeting key signaling pathways Tumor and blood vessels Another major class of targeted drugs is designed to disrupt critical signaling pathways in cells, especially signaling networks that control cancer cell growth. One of the pathways is controlled by the EGFR protein. The first EGFR drug was gefitinib, which was approved for treatment with NSCLC in 2003. Two years later, the FDA approved the second EGFR drug cetuximab for advanced colorectal cancer, and another similar drug, panitumumab, was approved in 2006. However, in 2008, new research revealed that colorectal cancer patients with mutations in the KRAS gene developed resistance to cetuximab and panitumumab. This finding requires routine testing of KRAS mutations to ensure that patients benefit from both drug treatments while protecting other patients from the adverse effects of unhelpful treatment. In 2004 and 2005, the FDA approved the EGFR inhibitor erlotinib for the treatment of NSCLC and advanced pancreatic cancer. Recently, in 2013, the US FDA approved the treatment of afatinib for patients with advanced NSCLC who carry EGFR gene-specific mutations. Other EGFR-targeted drugs are undergoing clinical trials. New HER2 therapy continues to break through breast cancer treatment About 15 years ago, scientists discovered the first treatment for tumor tissue that overexpressed human epidermal growth factor receptor 2 (HER2). Approximately 15% to 20% of breast cancer patients carry the above-mentioned genetic abnormalities (HER2-positive cancer). Similar to the EGFR of the same family, HER2 also promotes cancer cell growth. Since then, four HER2 targeted drugs have been born that can improve the survival of HER2-positive breast cancer patients. The combination of the first HER2 drug trastuzumab with chemotherapy significantly improves survival in women with advanced HER2-positive breast cancer. In 2006, trastuzumab was approved for early HER2-positive breast cancer patients with a view to reducing the risk of postoperative recurrence. Recently, an important study found that a double strike against HER2 was more effective than a single treatment with trastuzumab, which led the FDA to approve a second HER2 drug, pertuzumab in combination with trastuzum in 2012. Monoclonal antibodies are used in patients with advanced HER2-positive breast cancer and were approved for treatment in the early stages of the disease in 2013. In the same year, trastuzumab-emtansine (T-DM1) (conjugated with trastuzumab and a chemotherapeutic drug) was also approved. This combination therapy is not only more effective than single drug therapy, but it also allows the drug to be precisely targeted to breast cancer cells, thereby reducing the adverse effects on healthy tissue cells. This is the optimal treatment for HER2-positive breast cancer, which has worsened after several previous treatments. The fourth HER2 drug, lapatinib, was approved in 2007 and is effective in the treatment of HER2-positive and hormone-receptive/HER2-positive metastatic breast cancer when used in combination with aromatase inhibitors. Targeting drugs that act on multiple molecular pathways: broad prospects Researchers have consistently discovered that many cancer drugs can block multiple molecular targets or pathways simultaneously, making them a more effective anti-cancer tool. For example, vandetanib (approved for the treatment of thyroid cancer in 2011) Block EGFR, VEGFR (protein involved in tumor angiogenesis) and RET. The colorectal cancer drug regorafenib (approved in 2012) blocks six different cancer pathways: VEGFR1-3, TIE2, PDGFR, FGFR, KIT, and RET. New targets and new drugs The prospects for new drug development are extremely attractive. In 2013 and 2014, the FDA approved trimetinib and darafini, which are used in the treatment of BRAF-specific mutant melanoma, which controls the MEK pathway. Crizotinib (approved in 2013) targets lung cancer and childhood cancers that target ALK mutations. Tesirolimus (approved in 2007) and everolimus (approved in 2012) block the mTOR pathway, which controls the growth of several cancers, including breast, pancreatic and kidney cancers. Everolimus is the first effective targeted drug for HER2-negative breast cancer, which accounts for the majority of breast cancer. The combination of everolimus and aromatase inhibitors is approved for hormone receptor-positive, HER2-negative postmenopausal women with advanced breast cancer. Nilotinib (approved in 2007) and dasatinib (approved in 2010) can target BCR-ABL, a specific protein that can only be found in certain types of leukemia. Meeting the era of immunotherapy Scientists knew that the immune system was a powerful force against cancer as early as a hundred years ago. But it wasn't until the last decade that immunotherapy really began to revolutionize cancer treatment. Progress has been made in several directions, from oral medications to cell-based treatments tailored to each patient. Inspire the immune system to fight cancer T cells play an important role in cancer resistance. In 2011, the FDA approved Ipilimumab as a breakthrough treatment for melanoma. Ipimuzumab is an immunological drug that targets the T cell CTLA-4 protein, which inhibits the killing of T cells. In clinical trials, patients experience rapid and significant tumor regression and still benefit after a long period of treatment (which may last for years for some patients). Since then, some so-called immune checkpoint inhibitor drugs have been developed, especially some drugs can target the PD-1/PD-L1 pathway, which helps tumors escape the immune system. The FDA has awarded the PD-1 blocker drugs nivolumab and MK-3475 breakthrough therapy, both of which have shown unprecedented efficacy in recent clinical trials of melanoma (nivolumab is also effective for kidney cancer) And treatment of lung cancer). In September 2014, Mk-3475 (pembrolizumab) became the first FDA-approved PD-1 targeted drug. The PD-1 targeting drug MPDL3280A also showed an effect against advanced melanoma in clinical trials. Recent studies suggest that a combination of different checkpoint inhibitor drugs or an immunostimulatory drug such as interferon, interleukin, and other checkpoint inhibitor drugs may further improve patient benefit. Patients and survivors have significantly improved their quality of life Over the past decade, the study has discovered a range of new treatments that improve the quality of life for patients from diagnosis to survival. In addition, emphasizing the integration of early palliative care and active treatment will help many patients, especially those who can make advanced patients better. Relieve cancer-related adverse effects New strategies designed to control adverse effects can greatly improve a patient's quality of life, both during and after treatment. For example, two independent studies have shown that the antidepressant duloxetine and the antipsychotic olanzapine are effective drugs for preventing two common adverse effects of chemotherapy peripheral neuropathy and nausea. Another study found a treatment for common symptoms that did not receive enough attention - depression and pain. A growing body of evidence confirms the effectiveness of non-medical means such as acupuncture and yoga to improve the physical and mental health of patients and survivors. Possible benefits include relieving fatigue and pain, improving quality of life, and reducing drug use. Combination of cancer treatment with early palliative care A key clinical trial in 2010 demonstrated that integrated early palliative care in treatment significantly improved quality of life and prolonged survival for patients with advanced lung cancer compared with single active treatment. In addition, patients who have received early palliative care are less likely to receive high-intensity active care such as resuscitation at the end of the day. The study sparked a new wave of palliative care for advanced patients. The study also referred to the provisional guidelines recommended by ASCO in 2012: for any patients with metastatic or high-symptomatic burdens, palliative care can be associated with early standard cancer treatment. Common drugs that reduce the risk of cancer A large number of clinical trials have shown that some commonly used drugs may play an important role in cancer prevention. For example, analysis of data from nearly 50 epidemiological studies has shown that oral contraceptives reduce the risk of ovarian cancer by 20% every 5 years. This reduction effect persists within 30 years of discontinuation of the drug. Further research has found that taking aspirin daily can reduce the risk of colorectal cancer, but for the risk of stomach bleeding, it is not recommended to use aspirin as a preventive measure. The next step will also explore anti-inflammatory drugs in cancer prevention and The role of treatment. Another benefit of vitamins for the human body is that it can help the growth and development of the human body to operate normally, especially for teenagers, vitamins are one of the essential nutrients in the growth process. For example, vitamin D can well adjust the metabolism of some trace elements in the human body, and can also promote the absorption of calcium by the human body, maintain bone health, and maintain a balance between the blood phosphorus level and the blood calcium level in the human body. Vitamin Efficacy,vitamin drink,The benefits of vitamins,vitamin water YT(Xi'an) Biochem Co., Ltd. , https://www.ytherblifes.com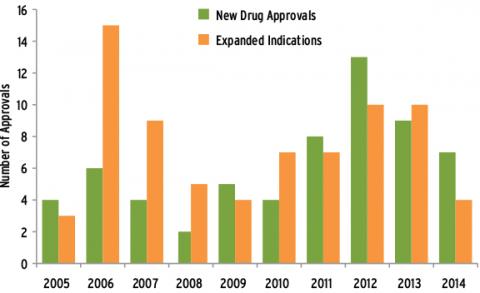
Figure 1 Summary of FDA-approved anticancer drugs for the decade (2014 to October) 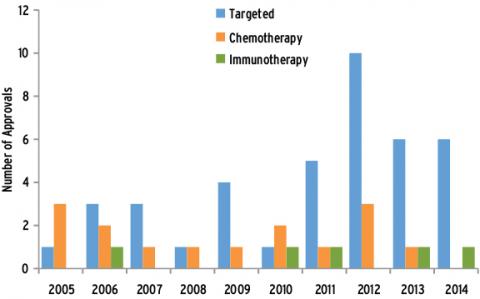
Figure 2 FDA-approved anticancer drugs in the last decade (2014 to October)