New trends in the detection of extracellular vesicles (cell microparticles, exosomes)
Extracellular vesicle Extracellular Vesicles (EVs) are vesicle-like bodies of a two-layer membrane structure that are detached from the cell membrane or secreted by the cells, ranging in diameter from 40 nm to 1000 nm. Extracellular vesicles are mainly composed of microvesicles (MVs) and exosomes (Exosomes, Exs), which are small vesicles that are detached from the cell membrane after cell activation, injury or apoptosis, and have a diameter of about 100 nm – 1000nm; The exosomes are released to the outside of the cell by exocrine forms after fusion of the multivesicular bodies in the cells with the cell membrane, and have a diameter of about 40 nm to 100 nm. Extracellular vesicles are widely present in cell culture supernatants and various body fluids (blood, lymph, saliva, urine, semen, milk), carrying a variety of proteins, lipids, DNA, mRNA, miRNAs associated with cell origin. Etc., involved in intercellular communication, cell migration, angiogenesis, and immune regulation. Elevated extracellular vesicles are found in diabetes, cardiovascular disease, AIDS, chronic inflammatory diseases, and cancer, and they are likely to be diagnostic markers for such diseases. Therefore, accurate characterization of extracellular vesicles is possible. And quantitative research is particularly important. At present, the detection methods of extracellular vesicles (EVs) mainly include scanning electron microscopy, atomic force microscopy, dynamic light scattering, nanoparticle tracking analysis (NTA), flow cytometry and ELISA, etc., due to high throughput and short time. Simple to operate, ELISA and flow cytometry are more common methods. The ELISA method is easily interfered by other soluble antigens, and it is impossible to know the size and quantity of vesicles; flow cytometry can not only detect the size and number of vesicles, but also detect the source of vesicles by cell-specific marker staining. The vesicles are classified. Therefore, flow cytometry is theoretically the best choice for rapid, high-throughput, multi-parameter detection of vesicles. However, the traditional flow cytometry is mainly for cells, the detection limit of scattered light is usually 300-500nm, and most extracellular vesicles are below 300nm in diameter. Because they cannot distinguish from background noise, the diameter is less than 300nm. Extracellular vesicles are difficult to detect, so the number of vesicles is often underestimated and the results are naturally inaccurate [1]. Three advantages of Apogee flow cytometry (1) The most sensitive scattering light resolution <br> Apogee's A50-Micro breaks the detection limit of traditional flow cytometry, optimized optical module and excellent scattered light detection capability make A50-Micro unmatched sensitivity (<100 nm) and the best light scattering resolution (10 nm). Figure 1 shows the comparison of the A50-Micro with the traditional flow cytometer (F500) and some new flow cytometers (Gallios, Influx) [1]. The forward-angle scattered light FC500 can only barely distinguish 0.5μm and 0.9μm beads, beads below 0.5μm are completely indistinguishable; Gallios and Influx have improved ability to detect scattered light, and can distinguish between 0.3μm and 0.5μm beads, but beads below 0.3μm still cannot. Differentiating; the Apogee A50-Micro can easily separate 0.14 μm beads and 0.3 μm beads into two groups (Fig 1.A). It can be seen from the scatter plots of the forward-angled scattered light and the side-angle scattered light that neither the FC500 nor the Gallios or Influx can partially separate the 0.3 μm beads from the background noise, while the A50-Micro can be clearly defined. The 0.3 μm beads were completely distinguished from the background noise (green data points in Fig 1. B), and only 0.14 μm beads were detected by A50-Micro (purple data points in Fig 1. B). These results demonstrate that with the high sensitivity and resolution highlighted by Apogee A50-Micro, we can easily cluster, count, and analyze extracellular vesicle samples with diameters greater than 10 nm. In addition, up to 9 channels of fluorescence detectors, flexible use of extracellular vesicle-specific antigen fluorescent antibodies for accurate analysis of vesicle source and quantity. Apogee A50-Micro is the only flow cytometer on the market that can detect small particles as small as 100 nm by scattered light, and is superior to any competitor in the detection of extracellular vesicles. **Diameter of a lipid or cellular microparticle with the same forward scatter relative intensity as the particle that was measured. Equivalent microparticle diameter (mm) = 3.2911(PD) - 0.2768, Where PD = polystyrene microsphere diameter (mm). Figure 2. Location of particles (180, 240, 300, 590, 880, 1300, 2000 nm) with refractive indices of 1.38 and 1.42, respectively, in a scatter plot. (3) Exosomal detection ability independent of antibody microbeads [1] Lacroix R, Robert S, Poncelet P, Dignat-George F. Overcoming limitations of microparticle measurement by flow cytometry. Semin Thromb Hemost. 2010 Nov;36(8):807-18. We're professional Others Gynecology Surgery manufacturers and suppliers in China, specialized in providing high quality medical instruments with reasonable price. We warmly welcome you to buy or wholesale bulk Others Gynecology Surgery for sale here and get quotation from our factory. Gynecology Forceps,Uterine Biopsy Forceps,Gynecology Suture Needle,Gynecology Surgery Instruments Tonglu WANHE Medical Instrument Co., Ltd , https://www.vanhurhealth.com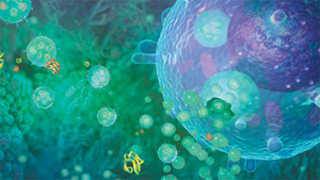
Method for detecting extracellular vesicles 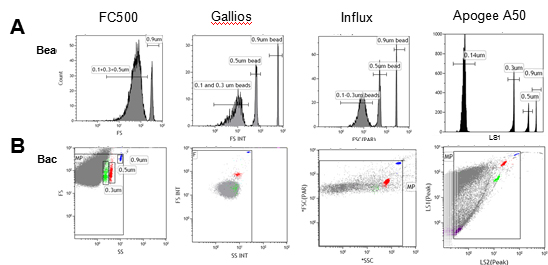
Figure 1. Technological improvement in forward scatter (FS) for microparticle (MP) measurement: (A) Beads resolution improvement: FS distribution of a blend of fluorescent latex beads (0.1/0.14, 0.3, 0.5 and 0.9 μm) with a threshold on (B) Background noise reduction: Scatters dot plot showing blue (0.9 μm beads), red (0.5 μm beads), green (0.3 μm beads) and violet (0.1/0.14 μm beads) beads with a FS threshold. Grey dots Are background noise.
(2) Patented refractive index correction function
The use of flow cytometry for extracellular vesicle detection often requires the use of standard microspheres to calibrate and set the gate. The commonly used microsphere materials are polystyrene and silica. The Megamix recommended by the International Society for Thrombosis and Hemostasis and the Standardization Committee (ISTH SSC) for the detection of cyclic microparticles (microvesicles) is a polystyrene microsphere. However, more and more researchers have recently found that there is a serious deviation in the setting of gates with standard microspheres, because the refractive index of microspheres of different materials is different, microspheres of the same diameter, refraction The greater the rate, the stronger the scattered light produced. Moreover, the refractive index of the microspheres is much larger than the refractive index of the biological vesicles, meaning that the intensity of the scattered light generated by the microspheres is several tens of times that of the same diameter vesicles. In fact, 0.5mm polystyrene microspheres (Megamix) produce scattered light intensity equivalent to 1.4mm vesicles, while 2.7mm vesicles produce scattered light of equivalent intensity to 0.9mm polystyrene microspheres (Megamix). (Table 1) [2]. Therefore, if we use a 0.5mm polystyrene microsphere (Megamix) to set the door to detect extracellular vesicles less than 0.5mm will cause serious deviation, the test results are also not credible. 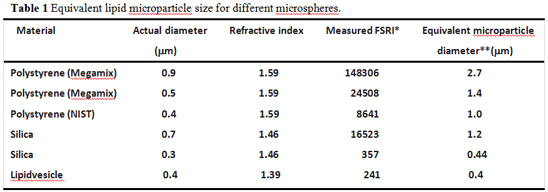
To solve this problem, Apogee engineers developed a patented refractive index correction function, whether it is a single-parameter Histogram or a two-parameter cytogram, just right-click and select “Choose Calibration Scaleâ€. A scaled gauge will appear, and the scale of the corresponding index of refraction can be selected based on the refractive index of the test sample (Fig. 2). Then we can set the gate and select according to the diameter and refractive index of the sample. The region of interest (ROI) is a reliable result. 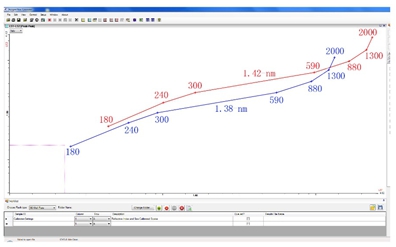
Due to the extremely small diameter of exosomes (40 - 100 nm), which is beyond the scope of traditional flow cytometry, many companies have provided specific antibody-labeled microbeads for exosome enrichment and Flow cytometry, but the limitations of this approach are complex operations, high antibody specificity, and the inability to know the absolute number of exosomes. The ultra - high sensitivity and resolution of the Apogee A50-Micro allows it to directly analyze and count exosomes in the sample. It is easy to operate and accurate. Caris Life Sciences, the leading biotechnology company in the US, is using the A50. -Micro to detect and analyze microvesicles and exosomes in complex patient samples [3]. The choice of first-class companies is worth your trust!
[2] Chandler WL, Yeung W, Tait JF. A new microparticle size calibration standard for use in measuring smaller microparticles using a new flow cytometer. J Thromb Haemost. 2011 Jun;9(6):1216-24.
[3] Abstracts from the Third International Meeting of ISEV 2014. Rotterdam, The Netherlands, April 30th – May 3rd, 2014. J Extracell Vesicles. 2014; 3:24214.
For more information on Apogee Flow Cytometry, please visit: Apogee Flow Cytometry