Google Verily non-invasive diagnostic system for non-invasive diagnosis of cancer
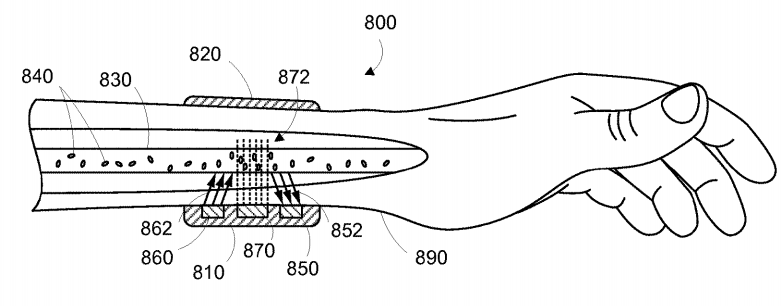
In April of this year, Verily launched the new health tracking wearable device Study Watch, and plans to use it on a large scale for medical research, mainly for the establishment of health databases and Parkinson's disease research. Recently, Verily has a new trend. According to the CBINSIGHT patent search engine, a new patent has just been authorized (patent application was approved in February 2014 and approved on May 8, 2017). A non-invasive diagnostic system that can be applied to a variety of diseases, including hormonal problems, infections, and even cancer. This diagnostic system monitors and evaluates certain substances that are inserted or ingested by the wearable device. By monitoring the type of particles that interact with the substance, the system can provide feedback to the device user about the potential disease risk. Schematic diagram of Verily's new patent system Magical "engineering particles" The patent that was freshly released in the first two weeks, specifically a contrast agent, is somewhat similar to the developer injected into the body prior to MRI. According to the patent description, the injectable material consists of "engineered particles" intended to interact with analytes in blood such as human antibodies. This interaction is then captured by the device on the user's wrist to achieve the diagnosis of multiple diseases. The official name of this patent is "engineered particles with polarization shrinkage and linear control for enhanced imaging." Engineered particles in the blood combine with antibodies, DNA, peptides, or other proteins that characterize underlying disease, and interact with engineered particles using electromagnetic waves that penetrate the skin to detect disease information. There are many ways to introduce engineered particles into the body, either by digestive tract ingestion, inhalation, injection, transdermal or a variety of other routes. The patent shows that the engineered particles can be specially prepared materials, or they can be molecular, viral or conductive nanorods, or even quantum dots, which are often referred to as "artificial atoms". It is also worth mentioning that these particles will be designed to remain in the vasculature or body fluids of the human body for a long time.
There are mainly the following parts:
1. Sampling swab with disposable sterile plastic rod/rayon head
2. Sterile sampling tube containing 3ml of virus maintenance solution (gentamicin and amphotericin B are selected to better inhibit the fungus in the sample. Avoid the human sensitization reaction that may be caused by penicillin in the traditional sampling solution.)
In addition, there are additional parts such as a tongue depressor, a biosafety bag, etc.
Intended to use
1. It is used for monitoring and sampling of infectious pathogenic microorganisms by disease control departments and clinical departments.
It is suitable for sampling of influenza virus (common influenza, highly pathogenic avian influenza, H1N1 influenza virus, etc.), hand, foot and mouth virus and other types of viruses. It is also used for sampling of Mycoplasma, Chlamydia, Ureaplasma, etc.
2. It is used to transport nasopharyngeal swab samples or tissue samples from specific parts from the sampling site to the testing laboratory for PCR extraction and testing.
3. It is used to preserve nasopharyngeal swab samples or tissue samples from specific parts for necessary cell culture.
The virus sampling tubes are loaded with infectious substances, and some are even highly pathogenic substances. Therefore, the requirements for packaging containers are very strict, and three requirements must be met at the same time:
1. The safety of transportation.
Ensure that the sample does not leak during transportation. Sampling tubes that comply with WHO regulations and biosafety regulations.
2. The security of preservation.
Ensure that the sample does not leak during storage. Sampling tubes that comply with WHO regulations and biosafety regulations.
3. The validity of the sample.
Make sure that the sampling tube itself will not have a toxic effect on the sample.
Virus Sampling Tube,Virus Sampling Kit,Disposable Vtm Sampling Kit,Vtm Sampling Tube With Swab Jilin Sinoscience Technology Co. LTD , https://www.contoryinstruments.com