"Nature Chemical Biology": Cope rearrangement and cyclization reaction structure basis in Hapalindole biosynthesis
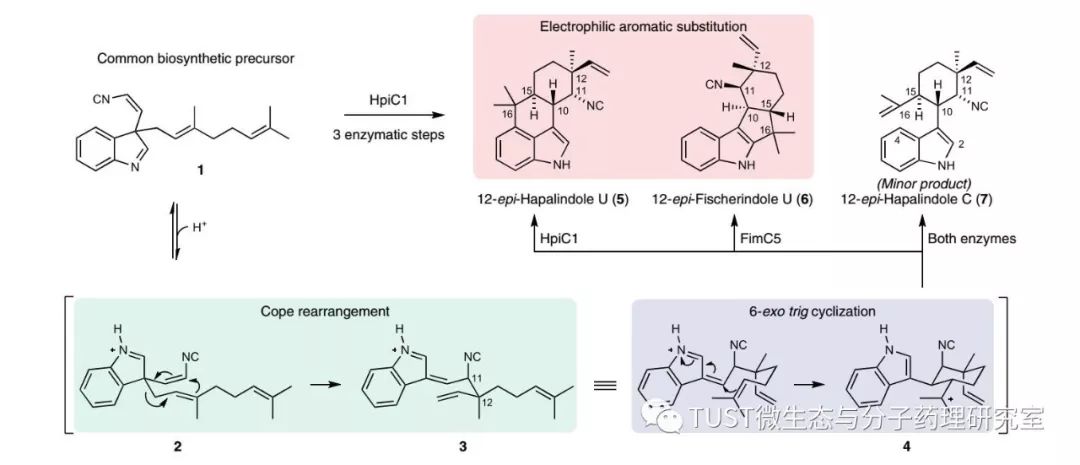
01 Introduction INTRODUCTION The Hapalindole alkaloid is a secondary metabolite produced by the genus Stigonematalean. It is a natural compound of a polycyclic system (å²å“š and monocyclic cyclization) with multiple chiral centers. , antifungal, anti-tuberculosis, insecticidal, anti-mitotic and anti-tumor and other biological activities. According to the structure of the multi-ring system, it can be divided into four categories: hapalindole, ambiguine, fischerindole and welwitindolinone. Due to the complexity and unique biological properties of the Hapalindole alkaloid structure, many studies have focused on the synthesis of these compounds. However, little is known about the biosynthesis of these alkaloids, especially the construction of the four-ring core system. Initial reports indicate that Hapalindoles are derived from cis-mercaptoonitrile and geranyl pyrophosphate (GPP), but the biological origin of the polycyclic system remains unclear. Sean A., School of Life Sciences, University of Michigan, USA Newmister et al. previously discovered a specific intermediate in the biosynthesis of Hapalindoles, a prenylenylated pseudoquinone, which undergoes a Cope rearrangement and then undergoes a cyclization reaction to form a product 12-epi -hapalindole U. Cope rearrangement is a [3,3]-sigma migration rearrangement, ie, a cyclical reaction of [3,3]-sigma migration. This pericyclic reaction is a multi-center reaction that is completed in one step by the formation of a transition state without the formation of an intermediate in the reaction. Although this pericyclic reaction is common in organic synthesis, it is rarely found in biosynthetic transformation. This polycyclic system structure is catalyzed by many stig cyclases. Although the homology between these cyclases is high, the products differ greatly. The study of this complex pathway of 12-epi-hapalindole U expands the understanding of stig cyclase, demonstrates the complex and diverse configuration of this class of alkaloids, and the stereochemical and regioselective mechanisms for the product. Related research is also very important. On this basis, they further studied the structure-activity relationship of the important stig cyclase, and published in 2018 in "Nature Chemical Biology" (IF = 13.99, Bio-region 1) entitled "Structural basis" Of the Cope rearrangement and cyclization in hapalindole biogenesis". 02 Main method used METHODS 1. Crystallographic method 2. NMR 3. LC–MS 4. Quantum mechanical calculation 5. Molecular dynamics simulation 6. Autodock VINA 03 Summary of the main contents of the article ABSTRACT The Hapalindole alkaloid is a structurally diverse natural secondary metabolite produced by cyanobacteria with different polycyclic systems and multiple biological activities. These complex metabolites are produced by a biosynthetic intermediate through three steps of Stig cyclase: Cope rearrangement, 6-exo-trig cyclization, and electrophilic aromatic substitution. A structure of the Stig cyclase HpiC1 capable of catalytically producing 12-epi-hapalindole U in vitro is reported herein. The resolved structure has a resolution of 1.5 angstroms and is a dimer structure in which each monomer contains two calcium ions, and the active site of the HpiC enzyme is located at the end of the protein dimer. Using amino acid mutation analysis methods and calculation methods, the authors revealed key amino acids associated with acid-catalyzed [3,3]-sigma migration rearrangements, as well as specific determinants that control the position of terminal electrophilic aromatic substitutions, ultimately leading to hapalindole to fischerindole The conversion of alkaloids. Portable Foam Fire Extinguisher Portable Foam Fire Extinguisher,6L Foam Fire Extinguisher,Powder Foam Fire Extinguisher,Foam Fire Extinguisher Nanjing Txfire International Trade Co., Ltd , https://www.txfireequipment.com