The medical network had two large varieties on March 1st, and the first one passed the consistency evaluation.
â–Levetiracetam sustained-release tablets
February 28, Salubris announcement that received levetiracetam release tablets (0.5g) approved by the State Drug Administration issued a "drug registration approval document."
According to the announcement, Xinlitai's levetiracetam sustained-release tablets were the first in China to be listed, and it was regarded as the consistency evaluation through generic drugs.
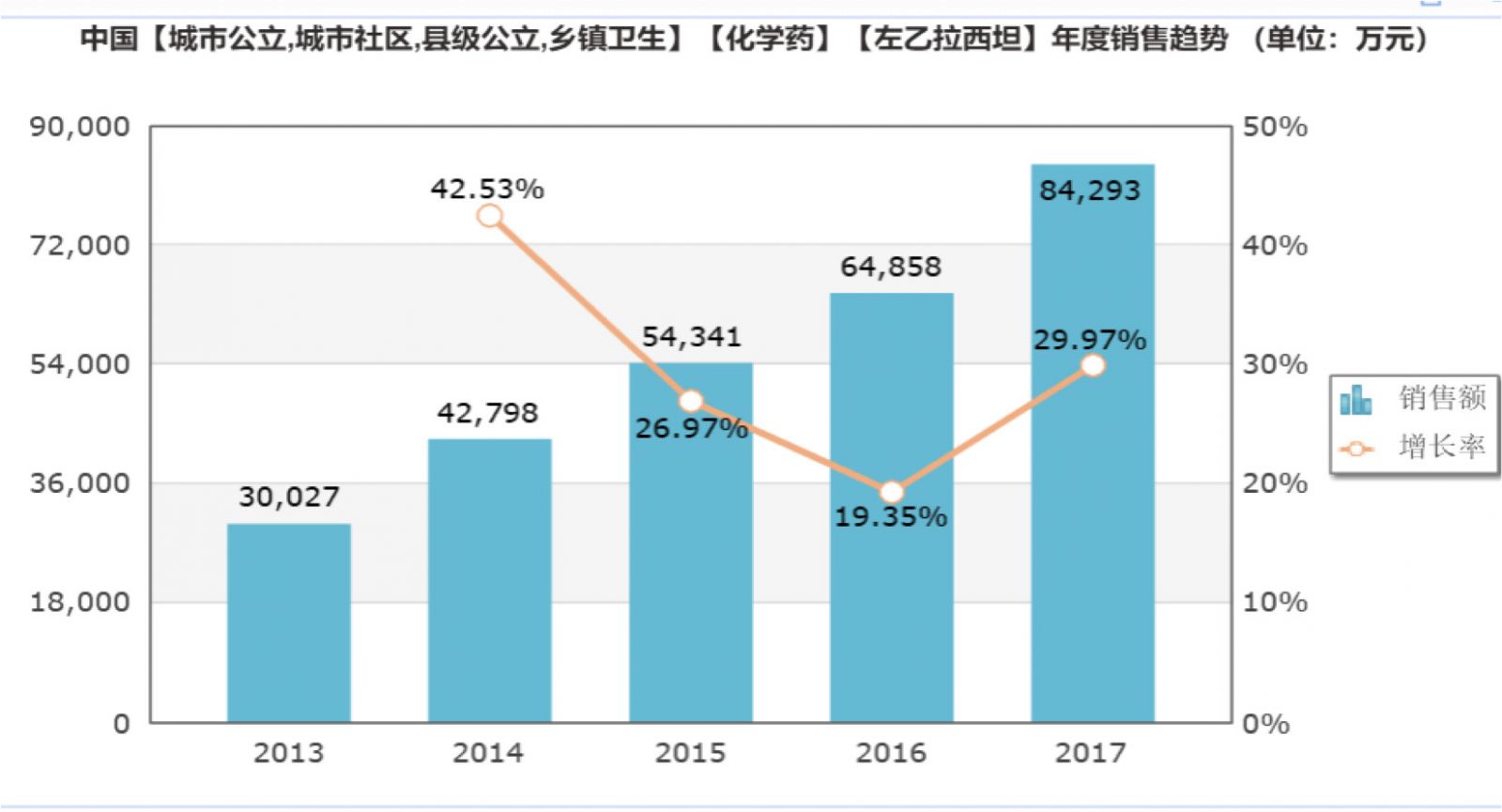
Market size: 842 million
It is understood that levetiracetam is a new anti-epileptic mechanism developed by Belgian Euphoria. It belongs to pyrrolidone-based sitan and is one of the main series of neurological drugs. It has fewer adverse reactions , lighter and better tolerance. .
According to the data from the intranet, in 2017, the sales of Lexiacetam in the national public hospitals was 842 million yuan, and the market growth rate was as high as 29.97%. In recent years, the growth rate has been above double digits. It can be seen that the demand and development potential of this variety market are very huge.
(Source: Minenet database)
First sustained release tablets listed
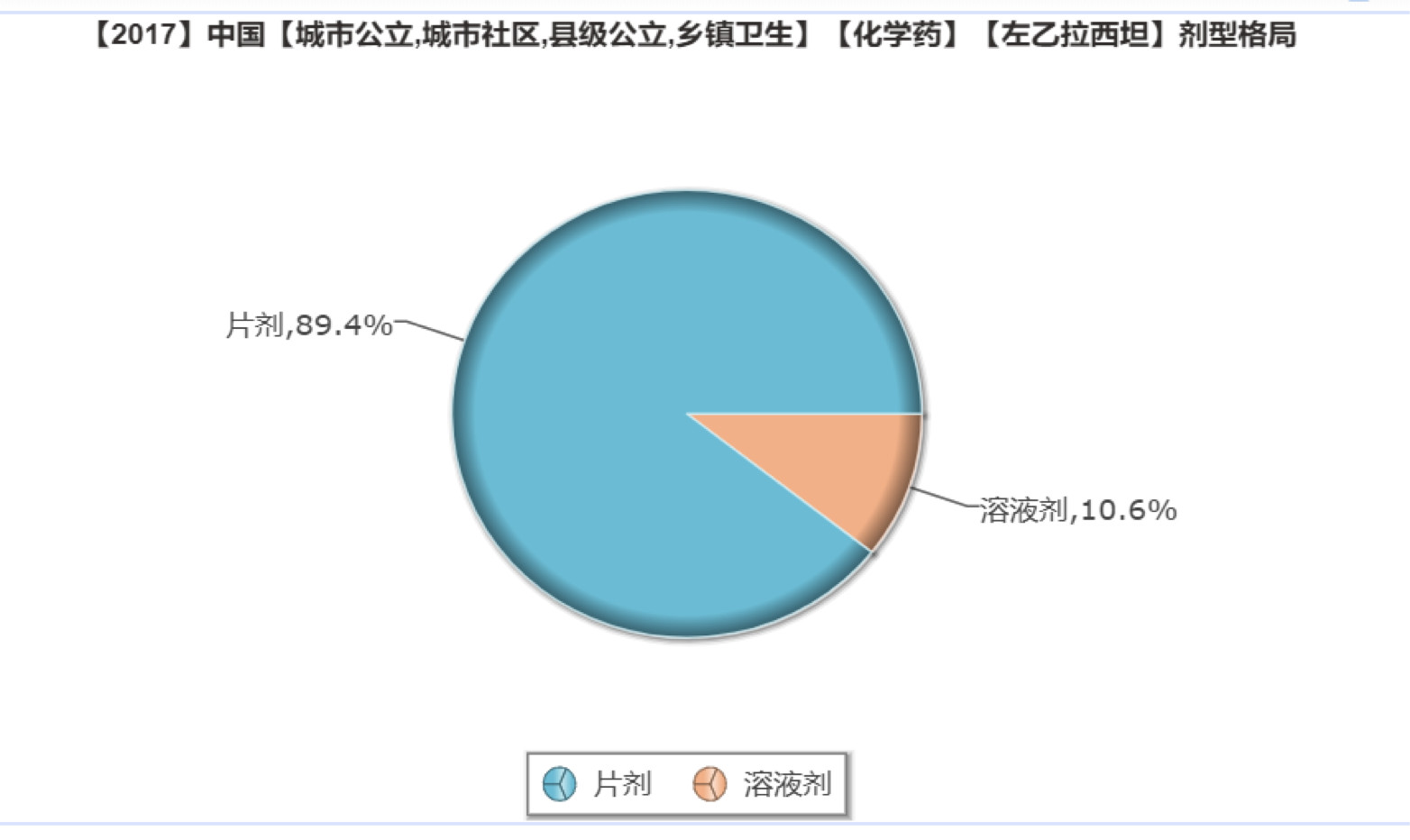
At present, the domestically marketed dosage forms are tablets, oral solutions, etc., and no sustained release tablets are available for sale. Xinlitai's levetiracetam sustained-release tablets are the first in China to fill the blank of similar dosage forms in China.
According to the intranet database, the current market for levetiracetam is mainly based on ordinary tablets, accounting for up to 89.4%; it is a solution, accounting for 10.6%. The approval of Xinlitai's sustained-release tablet type will bring about a certain change in the distribution of dosage forms currently available on the market.
(Source: Minenet database)
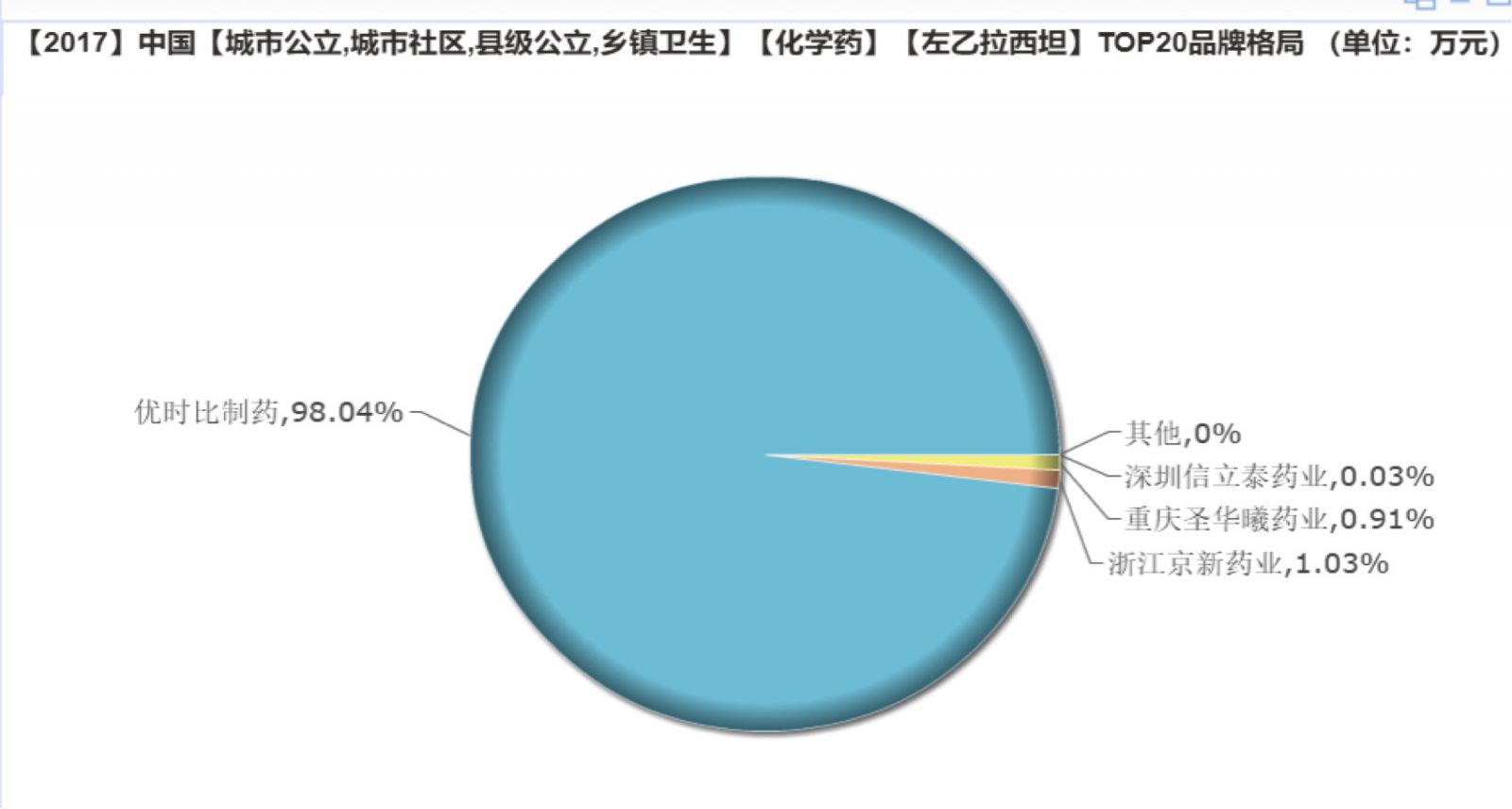
Market pattern: the original research one big
In terms of market structure, levetiracetam original research enterprise to work independently, high market share than when the original research enterprise excellent 98.04%, the rest of the market split between Salubris, Jingxin, San Joaquin Xi and other domestic pharmaceutical companies. Xinlitai's sustained-release tablet type was approved for listing, and it is considered to pass the consistency evaluation. It is believed that the future market pattern of the variety will have a certain impact.
(Source: Minenet database)
Trimetazidine hydrochloride sustained-release tablets
On the same day (February 28th), the official website of Jinan High-tech Zone released a news. Recently, the Food and Drug Administration officially approved the registration application of Qilu Pharmaceutical's sustained release tablets of trimetazidine hydrochloride.
According to reports, Qilu Pharmaceutical's trimetazidine hydrochloride sustained-release tablets were the first domestically marketed for this formulation, and it was deemed to have passed the consistency evaluation.
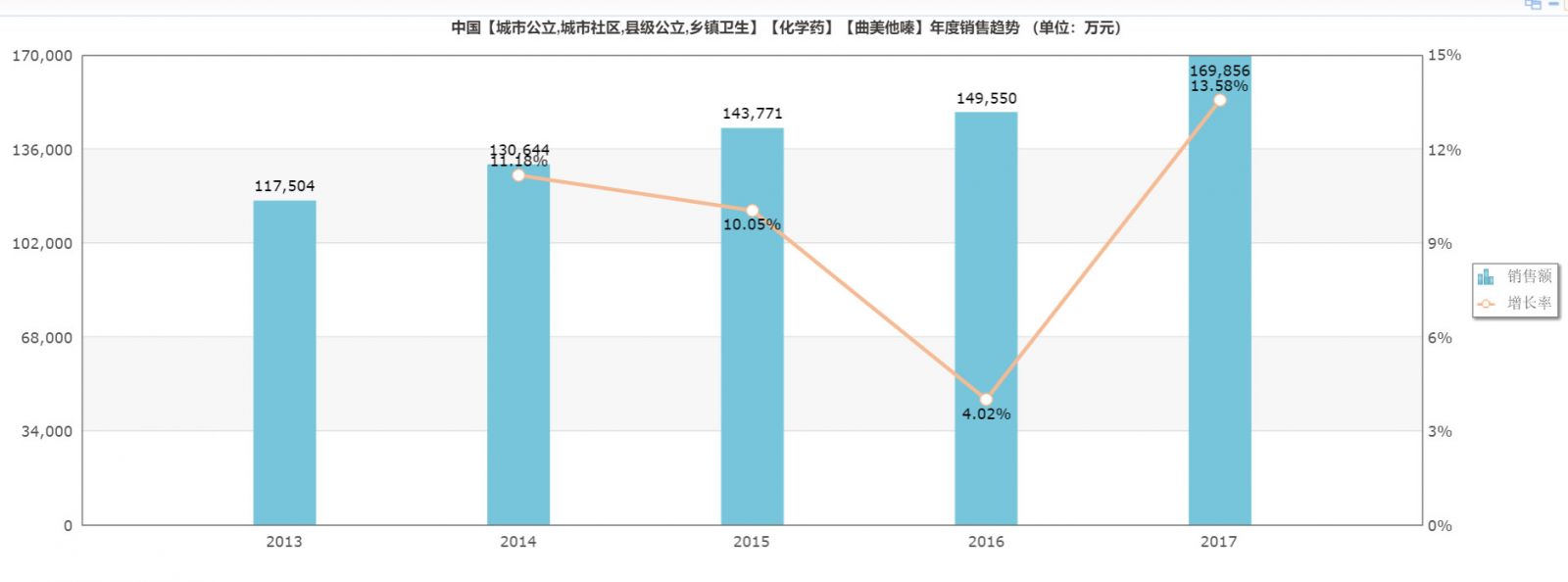
Market pattern: 1.699 billion
It is understood that trimetazidine is clinically suitable for stable angina with controlled or unacceptable first-line anti-angina therapy. By regulating myocardial energy metabolism, this product can effectively improve myocardial ischemia in patients with angina pectoris and significantly prevent recurrence of angina pectoris. The product has good tolerance, high safety and good patient dependence, and has been widely used in clinical practice.
According to the data from the rice network, the sales of trimetazidine in the national public hospitals in 2017 was 1.699 billion yuan, and the market growth rate was 13.58%. From the growth situation in recent years, the market growth of this variety is relatively stable.
(Source: Minenet database)
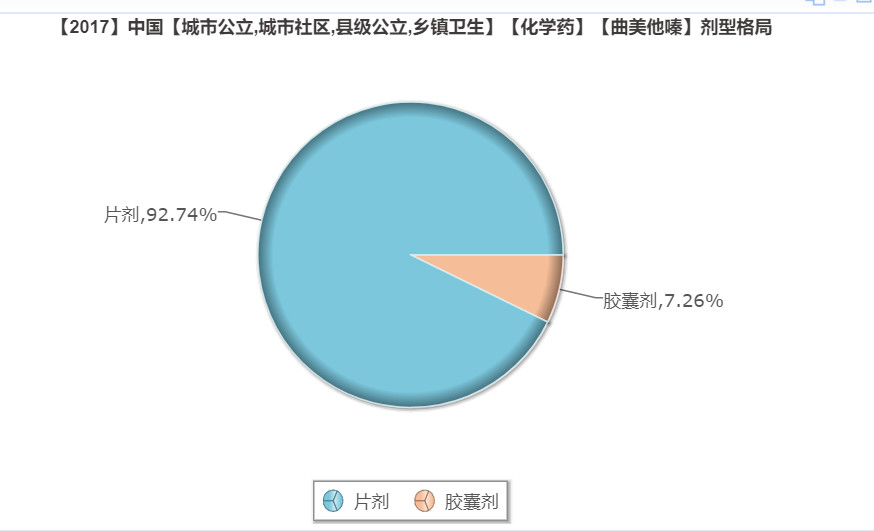
Dosage form: mainly tablet
According to the intranet database, the dosage forms of trimetazidine sold in public hospitals nationwide are mainly tablets and capsules, and there is no sustained release tablet type. Qilu Pharmaceutical's sustained-release tablets of trimetazidine hydrochloride were approved for marketing, which filled the gap in the market for this dosage form and will have a certain impact on the distribution of dosage forms in the current market.
(Source: Minenet database)
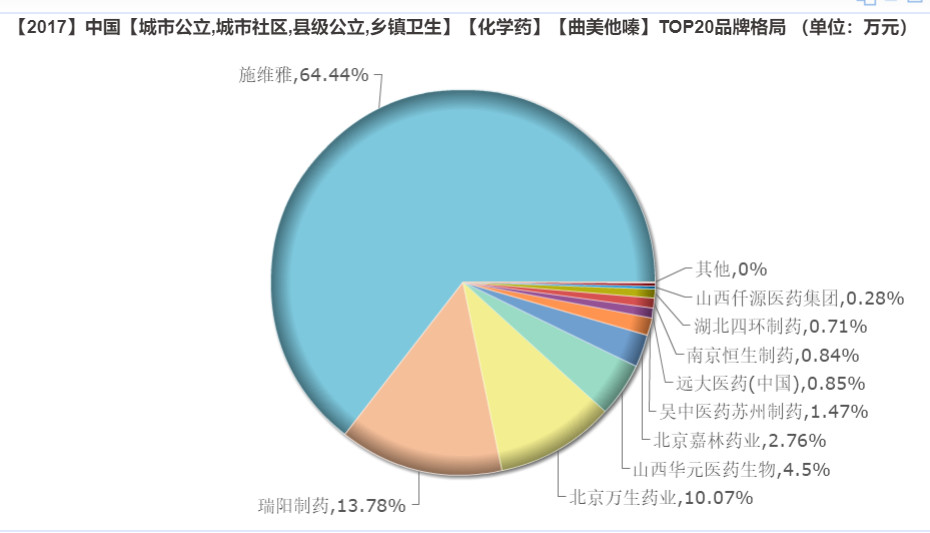
Competition pattern: domestic drugs launch shock
In terms of market structure, trimetazidine has a market of 64.44%, still occupied by the original research firm Servier. However, at present, domestic pharmaceutical companies are also exerting strength, and Ruiyang Pharmaceutical and Beijing Wansheng Pharmaceuticals all occupy more than 10% of the market.
Qilu Pharmaceutical's sustained-release tablets of trimetazidine hydrochloride were approved for marketing, which filled the market gap of the sustained-release dosage form of the variety, and will also have a certain impact on the existing market competition pattern in the future.
(Source: Minenet database)
Our mission: To protect the health of ear, throat and nose with medical frontier knowledge and technological innovation.
Our vision: Leader in several niche markets of Otolaryngology.
Our values: Change, Enterprising, Share