How does the clean room prevent cross-contamination?
How does the clean room prevent cross-contamination? How does the clean room prevent cross-contamination? The Code for Design of Clean Plants, which was implemented in 2002, clearly states that “the layout of the process plant should be reasonable and compact. Only necessary process equipment and processes and workshops with air cleanliness requirements should be placed in the clean room or clean areaâ€. Attachment: Prevention of errors, pollution and cross-contamination are the core of GMP*. Cross-contamination refers to the mixing of components of different varieties of drugs through pollution, such as personnel round-trip, tool transportation, material transfer, air flow, equipment cleaning and disinfection, post clearance, etc., or improper due to human, work tools, materials, air, etc. The flow direction causes contaminants in areas with low cleanliness to pass into areas with high cleanliness, causing cross-contamination. Sodium Fluoride CAS No.7681-49-4 Sodium Fluoride Basic Information Sodium Fluoride Anticoagulant,Sodium Fluoride Allergy,Sodium Fluoride 99,Industrial Grade Sodium Fluoride ShanDong YingLang Chemical Co.,LTD , https://www.sdylhgtrade.com
â– Improve the material level, processing accuracy, tightness and management system of equipment level equipment are related to cross-contamination. Therefore, in addition to reasonable layout, improving the automation level of equipment and composing the production line to reduce the operator and reduce the frequency of personnel activities is a necessary measure to prevent cross-contamination. The solid preparation workshop has a large amount of dust. How to prevent cross-contamination in solid preparation workshops? * First, the equipment should be equipped with a protective cover and carrying a dust removal device; secondly, isolation measures should be taken to divide it into an operation room and an front room or an operation room and an auxiliary machine room. The front room is arranged in a single plane in a plane layout, the auxiliary machine room can be located in a non-clean area, and the inspection door is located on the side of the corridor*. Such separation methods can be used for equipment such as tableting, automatic coating, and capsule dispensing. For some single machines that are not sealed with dust removal auxiliary equipment, such as pulverizer, powder or granule packing machine, the exhaust air in the isolation zone can be filtered and sent back to the isolation zone to form a self-circulation. In the production process, some drugs have strong wettability. When the relative humidity of the air is required to be less than 50% or even 45%, it is difficult to achieve the requirements of freezing and dehumidification. Among many dehumidification measures, lithium chloride rotary dehumidification is more suitable. The dehumidifier can be installed in a clean room with special dehumidification requirements, and the purified air is used as the low-humidity protective air of the post, which is a self-contained circulation system.
■The air conditioning purification system of the air conditioning purification system clean room should be divided according to different cleanliness levels. For studios of β-lactams, contraceptives, hormones, virulent microorganisms, anti-tumor drugs, radioactive drugs, etc., air-conditioning purification systems should be installed, and high-efficiency filtration equipment should be installed at the exhaust vents to contaminate these drugs. Down to a low limit. For clean rooms with different cleanliness levels, clean rooms that generate dust and harmful gases, and those with high toxicity and flammable and explosive gases should be equipped with local exhaust systems. The air outlet of the clean room should have an anti-backflow device. There should be interlocking devices for the opening and closing of air supply, return air and exhaust air.
â– Strictly control the flow of people's logistics. The clean room should have a dedicated flow of people and logistics. Personnel should enter according to the prescribed purification procedures and should strictly control the number of people. For the material, the dust can be removed after the dust is removed and sent out through the buffer room or the transfer cabinet. Items in clean areas of different cleanliness are delivered through the transfer window. The intermediate station should be located at the center to shorten the transport distance. There are no pipes in the clean area that are not related to this position. Make full use of the technical interlayer above or below or around, the main pipes of all common pipes and process pipes are installed in the technical interlayer. Pipes that pass through the ground and partition walls should be placed as close as possible to the point of use and the casing should be laid. There should be no welds in the pipes in the casing, and there should be a sealable seal between the pipe and the casing. The pipes entering the clean room should be made of stainless steel.
â– Reasonable layout of the space area reasonable layout * First straighten out the process, to avoid round trips. The plane space of the studio should be reasonable, which is beneficial to both operation and maintenance. The unused area and space should not be reserved. Reasonable space and area are also conducive to reasonable partitioning to prevent mixed accidents.
It should be noted that the clean room is not the bigger the better, the size of the area and the space are related to the amount of air supply, which determines the energy consumption of the air conditioner and affects the investment of the project. However, the space of the clean room should not be too small, too small may not be easy to operate and maintain. Therefore, the reasonable design of the space should take into account the needs of equipment operation and maintenance. Production areas and storage areas should have a space area that is compatible with the scale of production, used to house equipment and materials, and is easy to operate and maintain. *The clean room height is controlled at 2.60 meters, which can be locally increased for individual higher equipment, and it is not suitable to increase the height of the clean area. An intermediate station of materials should be provided inside the workshop, which is large enough to store materials, intermediate products, products to be inspected and finished products, and is easy to define the partitions to minimize errors and cross-contamination.
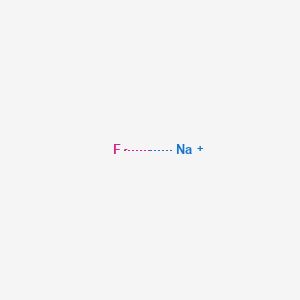
Product Name: Sodium fluoride
CAS: 7681-49-4
MF: FNa
MW: 41.99
EINECS: 231-667-8
Mol File: 7681-49-4.mol
Sodium Fluoride Chemical Properties
Melting point: 993 °C(lit.)
Boiling point: 1700 °C
Density: 1.02 g/mL at 20 °C
Vapor pressure: 1.4 mm Hg ( 0 °C)
Fp: 1704°C
Storage temp.: 2-8°C
Solubility H2O: 0.5 M at 20 °C, clear, colorless
Form: powder
Color: White to off-white
Specific Gravity: 2.558
PH: 7.0-10.0 (25℃, 0.5M in H2O)
Odor: Odorless
Water Solubility: 4 g/100 mL (25 ºC)
Sensitive: Hygroscopic