Hollow fiber tangential flow filtration purification of HSV vaccine
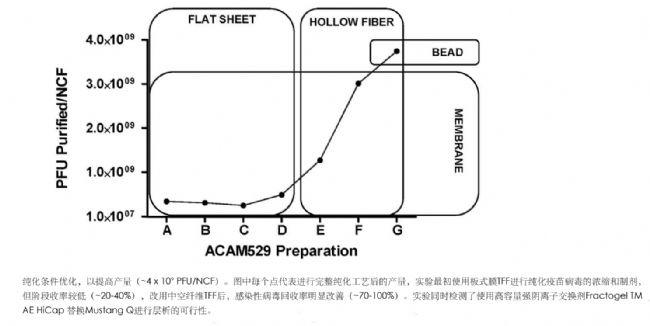
Introduction Herpes simplex virus type 2 (HSV-2) is the main cause of ulcerative genital herpes. There are 23 million new infections every year in the world. The treatment is mainly based on oral antiviral therapy, but HSV-2 vaccine has entered the clinic. Tests, including inactivation, activity decay, subunits, DNA and peptide vaccines, etc., wherein the wild type virus deletes the replication-defective HSV-2 vaccine strain virus constructed with the UL5 and UL29 genes (dl5-29 is re-derived and renamed ACAM529 It is considered to be a more effective candidate vaccine and effectively induces a protective immune response in both mice and guinea pigs. However, the traditional use of vaccines is prepared by centrifugal purification, which is not suitable for commercial production. The preparation of clinical viral vaccines includes upstream virus production and downstream virus purification, which requires maintenance of viral infectivity and clearance of host cell impurities. There are a variety of methods for purifying cell culture-derived virus particles. This experiment uses membrane chromatography combined with hollow fiber tangential flow filtration (TFF) to prepare a high-purity, functional ACAM529 vaccine. The main steps include the use of glucose sulfate (DS) from infection. The complementary Vero cell culture is eluted with ACAM529, endonuclease treatment, deep filtration, anion exchange chromatography, hollow fiber tangential flow filtration, and the obtained vaccine strain has high purity and good immunogenicity. experiment The Auro529 main virus seed bank was prepared by the existing method using the Vero cell line AV529-19. Small-scale culture production using 12-well plates and T125 shake flasks, then scaled to a 10-layer NUNC cell factory (NCF, working volume 2L, culture area 6320cm 2 , Thermo Fisher), optimized for culture conditions to ensure maximum ACAM529 yield . The experiment used two methods to purify ACAM529. The first method used planar membrane-packed tangential flow filtration combined with sucrose cushion ultracentrifugation. The specific procedure included manual scraping of infected cells from NCF, centrifugation, resuspension with stable buffer, and cell suspension. was added microfluidizer machine (Microfluidics Corporation) and the cells were mechanically disrupted cells cut genomic DNA, and then added to the Benzonase ® endonuclease (Merck Millipore) after treatment, clarified by centrifugation. The clarified cell lysate was concentrated from 1100 mL to 50 mL using a filtration system equipped with three 30 kD, 50 cm 2 polyethersulfone plate membranes. During the filtration process, the inlet pressure was maintained at 30 psi and the back pressure was increased from 1 psi to 8 psi to achieve sufficient The flow rate. The filtered reflux was subjected to ultracentrifugation with a 25% sucrose cushion for 4 h (50,000 x g, 4 °C), and the obtained ACAM529 particles were resuspended in 20% sucrose stabilization buffer and stored at -80 °C. The second method uses membrane chromatography in combination with hollow fiber tangential flow filtration. In the specific operation, firstly pour out the infection medium in NCF, add DS elution buffer, clarify by centrifugation after incubation, add Benzonase ® reaction solution, gently stir, and react for specific time, then use 0.65μm depth filter to remove residual Cell debris and other aggregated impurities. Membrane chromatography using Mustang® Q filter (Pall Corporation), sterilizing, pre-wetting, low salt balance before operation, rinsing with equilibration buffer after loading, then eluting impurities with 0.7 M NaCl buffer, and buffering with 2M NaCl ACAM529 containing fraction was eluted, which uses KrosFlo ® developed by tangential flow filtration system II i (Spectrum Laboratories, Catalog No: SYR2-U20-01N; with 100kD, 85cm 2 polysulfone hollow fiber filter assembly) and concentrated 5-10 Times, and 3-5 volumes of washing and changing to 20% sucrose stabilization buffer. During the operation, to reduce shear, the flow rate was set to 130 mL/min (cutting was approximately 4,000 S -1 ). When washing, the transmembrane pressure (TMP) is controlled to be below 4 psi to slow the gel layer formation. The product was stored at -80 °C. All operations were carried out under aseptic conditions, and the final product was not sterile filtered because of the large HSV-2 virus particles (180-200 nm). After purification of the product, the ACAM529 titer was determined, and the purity of ACAM529 was determined by ELISA, qPCR and PicoGreen dsDNA analysis, and the immunogenicity was analyzed by subcutaneous immunoassay in mice. result When the ACAM529 laboratory scale preparation was performed by sucrose cushion ultracentrifugation, there were 2 μg or more of host cell DNA residues per 1 X 10 7 PFU ACAM529, and when using the second method, the residual amount was less than 10 ng, such as host cells. Protein residue is a measure. The purity of the product is 200 times higher, and the purity of the process liquid is 2 orders of magnitude higher. When mechanical cell disruption occurs, there is a higher dsDNA residue in the product. Alternative is to use DS, wash the active chemical from the host cell surface extract obtained ACAM529, and provided with Benzonase ® endonuclease reduced the amount of DNA contained in the product. After comparing a series of chromatographic methods, it was found that the use of Mustang ® Q membrane chromatography cartridges yielded the highest yields, while the KrosFlo ® hollow fiber tangential flow filtration system replaced the membrane membrane filtration system, effectively doubling the yield. The reason may be that the open flow path reduces the shear, and the screen for forming the turbulent flow is added to the plate film design, although the mesh can increase the flow rate to a certain extent and reduce the formation of the gel layer, but at the same time increase Shear force on the sample. As measured by the Vero host cell protein (HCP) in the product, ACAM529 final product purification factor can be up to 250 times, and Vero DNA content is also lower than the WHO limit. Purification added during the DS, Benzonase ® the like may be respectively cleared depth filtration and membrane chromatography process. In addition, animal immunization experiments showed that the ACAM529 obtained by chromatographic purification has the same immunogenicity and protection as the sample obtained by centrifugation of the sucrose cushion. discuss ACAM529 is a prophylactic vaccine against replication defects that has been shown to be effective in preliminary animal experiments, but to meet further animal and clinical laboratory needs, there is an urgent need for a scale-up downstream purification process. The method used in this experiment combined with DS elution, Benzonase ® treatment, deep filtration, membrane chromatography and hollow fiber ultrafiltration / washing filtration, effectively improved the overall vaccine yield. It was found in the experiment that the hydrodynamic shear pressure is very important for the loss of infectious virus titer. Therefore, the closed channel plate membrane TFF and the bead chromatography were replaced with the hollow fiber TFF and membrane chromatography of the open channel. Higher infectious virus yields are obtained at each stage of the purification step. The vaccine immunogenicity and protective efficacy data obtained in animal experiments also confirmed the feasibility of the process. The content of this article is the editor's translation. If there is any inconvenience, please forgive me. For details, please refer to the original text. Original: Mundle, ST, Hernandez, H., Hamberger, J., et al., High-Purity Preparation of HSV-2 Vaccine Candidate ACAM529 Is Immunogenic and Efficacious In Vivo. PLOS ONE, 2013, 8(2): 1-10.
Xi'an Natural-Field Bio-technique Co., Ltd is a technology-driven enterprise, who dedicates to the research, production and selling of health-care raw material, cosmetic ingredients and plant extract. Over the years, our company positions at producing high quality, fine technique, cutting-edge products, adheres to the concepts of asset-light, high technology and team work principle, with scientific management methods and strict products standards, making great progress in the health-care field. Hot Selling powder,Hot Selling extract,Hot Selling Cosmetic ingredients,Hot Selling extract, Hot Selling health care ingredients Xi'an Natural Field Bio-Technique Co., Ltd. , https://www.naturalnf.com KrosFlo Research 2i [KR2i] Tangential Flow Filtration System
KrosFlo Research 2i [KR2i] Tangential Flow Filtration System