Development of a systematic method for the detection of ultra-high performance convergent chromatographic conditions using opioids
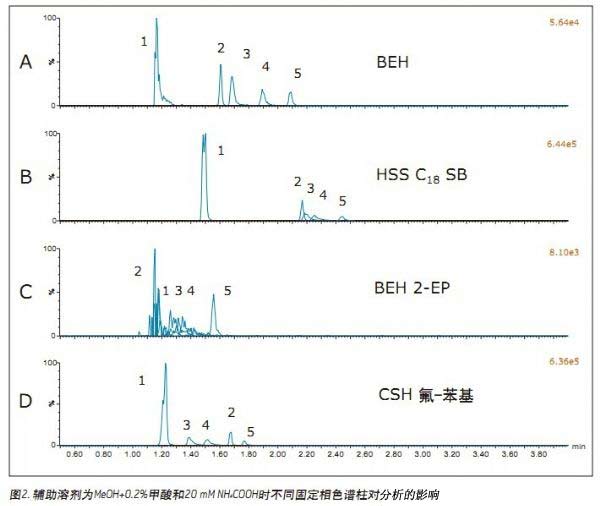
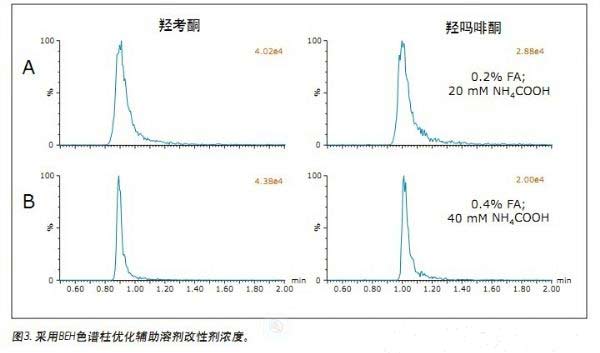
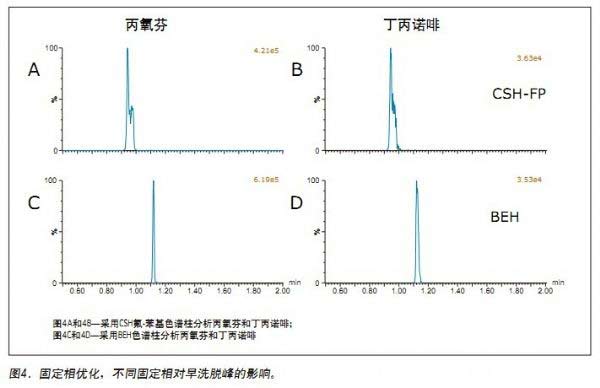
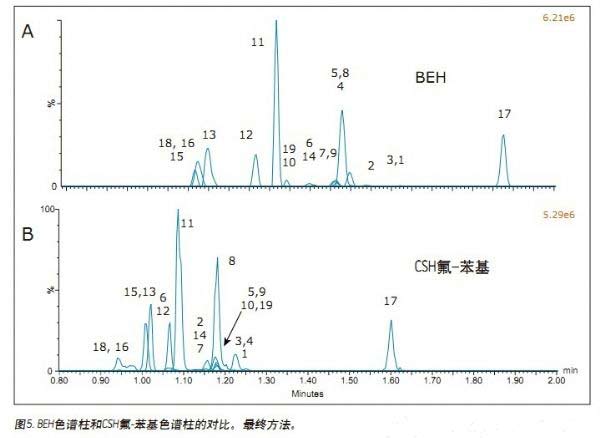
Jonathan P. Danaceau, Kenneth J. Fountain, and Erin E. Chambers APPLICATION BENEFITS Waters Solutions Key words <br>Opioids, pain management, UPC 2 /MS, method development, UPC 2 , Convergence chromatography Introduction <br>UltraPerformance Convergence ChromatographyTM (UPC 2 TM) is an innovative technology that fully applies the benefits of UPLC® to supercritical fluid chromatography (SFC). UPC 2 is an analytical technique orthogonal to UPLC that combines a renewable “green†solvent CO 2 with a high diffusion coefficient with a variety of complementary stationary phases to solve many of the problems encountered with conventional LC or GC analysis. Separation problems. Since most analytical chemists lack sufficient knowledge of UPC 2 , it is necessary to provide a direct development strategy for the development and optimization of UPC 2 methods. This application note highlights a systematic approach to the development of four different commercially available stationary phases combined with four different organic auxiliary solvents, which can quickly determine the optimal initial conditions for method development. After initial screening, an analytical method for 19 natural, semi-synthetic and synthetic opioids and related drugs was established by direct and rapid method optimization, which can be used for pain management, addiction treatment and drug abuse monitoring. The final method was able to complete the analysis of all compounds in 2 min and obtain acceptable peak shapes with a total cycle time of 4 min. experiment UPC 2 System <br> condition: ACQUITY UPC 2 Results and discussion The preliminary evaluation results for the additives on the BEH column are shown in Figure 1. All compound concentrations were 500 ng/mL. The spectrum clearly shows that oxycodone, oxymorphone and morphine have a broad MS peak shape and a low peak intensity when pure methanol is used and 0.2% formic acid is added to methanol. In contrast, when 0.2% NH4OH or 0.2% formic acid + 20 mM NH4COOH was used as an additive, the peak shape of most of the compounds (the compounds used in this initial screening) was acceptable. Under alkaline conditions, the analysis of basic compounds can obtain good chromatographic performance, which is particularly evident in the analysis of opioids under SFC conditions. The above results are consistent with previous reports. 1,2 By carefully analyzing the two chromatograms below, it is known that the buffer salt additive can obtain better retention properties and peak shape than the additive with only concentrated ammonia. Conclusions <br>This application uses a set of natural and synthetic opioid analyses to demonstrate a system screening strategy for UPC 2 method development. At the same time, a variety of different chemical properties of the column and a variety of different auxiliary solvents and additives can be quickly determined to determine the preferred initial conditions for further optimization. Simple and rapid conditional optimization established a method for the analysis of 19 different opioids in 2 min, with good retention times and peak shape results for all compounds. In addition, this approach further highlights the potential applicability of UPC 2 for the analysis of a wide variety of compounds.
Our company has a large supply of nutritional supplements, a wide variety of reliable quality,Nutritious supplementary,5-Aminolevulinic acid phosphate,Spermidine Trihydrochloride,Galantamine Hydrobromide,
We are a large group company in China and we are happy to share with you today our hot selling products, starting with organic extracts . Organic extracts are products derived from fruits, flowers, vegetables and whole foods. Organic extracts are rich in nutrients, including amino acids and vitamins. Organic extracts have a strong consumer base in the healthcare and cosmetics industries. The plant extracts contain beneficial phytochemical supplements for human health and act as natural antioxidants. next is Food Peptide .Food peptides are extracted from food by various processing or enzymatic hydrolysis methods, and the large food protein is converted into small molecular peptides. These peptides not only have very small molecular weight, but also have many biological functions, so we call them active peptides .next is plant extracts . Plant extracts refer to substances extracted or processed from plants (all or a part of plants) with appropriate solvents or methods, which can be used in pharmaceutical industry, food industry, daily chemical industry and other industries. next is Food Additives . Food additives refer to synthetic or natural substances added to food for the purpose of improving food quality, color, aroma and taste, as well as for the purposes of preservative and processing. next is Sweeteners are food additives that sweeten soft drinks. Sweeteners can be divided into nutritional sweeteners and non-nutritional sweeteners according to their nutritional value. According to its sweetness, it can be divided into low sweetness sweetness and high sweetness sweetness I flavor; According to its source can be divided into natural sweeteners and synthetic sweeteners .next is Healthcare products include apis, pharmaceutical equipment, etc.,
Nutritious supplementary, 5-Aminolevulinic acid phosphate,Spermidine Trihydrochloride, Galantamine Hydrobromide Xi'an Hollysince Biotech Co., Ltd. , https://www.hollysince.com
Waters Corporation (Milford, MA, USA)
â– System UPC 2
■Method development strategy ■Alternative selection of reversed-phase chromatography ■Fast analysis speed ■Excellent polar analyte retention performance ■“Green†mobile phase
ACQUITY UPC 2 TM System
ACQUITY UPC 2 Column
ACQUITY® TQD Mass Spectrometer
MassLynx® software
This paper applies UPC 2 to the analysis of a set of natural and synthetic opioids to demonstrate the development of the UPC 2 method. These compounds represent an important class of clinical drugs. Related applications include laboratory drug testing, pain management monitoring, and compliance with drug treatment protocols. Except for a report published about 25 years ago, 1 we have not found any reports of the use of SFC for the analysis of opioids. Therefore, we decided to evaluate UPC 2 by analyzing such important compounds.
Practicality.
sample discription
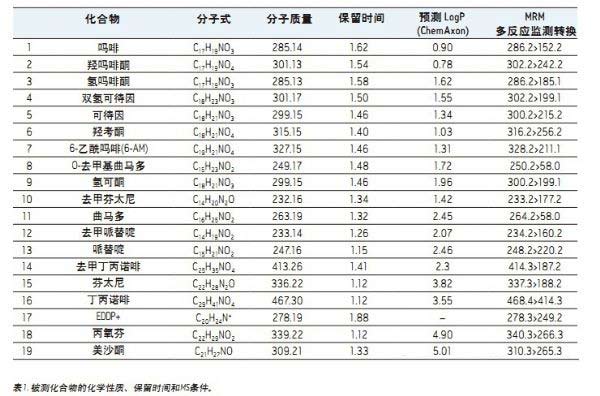
The 19 screened compounds are listed in Table 1 and constitute a comprehensive group of experimental drugs, including natural opioids for pain management, semi-synthetic opioids, and synthetic anesthetic analgesic compounds. Most of these compounds are weakly basic and have a pKa of about 8-9. They have a wide range of polarities, and the LogP values ​​range from 0.78 for oxymorphone to 5.0 for methadone, as shown in Table 1. The MRM multiple reaction monitoring conversion is shown in Table 1. Stock solutions of all compounds were prepared using methanol, and the working solution was methanol or isopropanol or acetonitrile/methanol (60:40). All compound concentrations in the working solution were 500 ng/mL.
Column: ACQUITY UPC 2 BEH, 2.1 × 50 mm, 1.7 μm (p/n 186006558)
Column temperature: 55 °C
Injection volume: 2μL
Flow rate: 1.5 mL/min
Mobile phase A: CO 2
Mobile phase B: Methanol vial with 0.4% formic acid and 40 mM NH4COOH: LC/MS certified 12 x 32 mm screw maximum recovery (p/n 600000749 CV)
Gradient: The initial condition is 2% mobile phase B. Mobile phase B increased to 50% in 2 min and 0.5 min after reaching 50%. The mobile phase B then returned to 2% in 0.1 min and the system was rebalanced for 1.4 min. The entire cycle time is 4.0 min.
MS mass spectrometer conditions <br>: ACQUITY TQD
Compensation pump flow rate: methanol with 1% formic acid (0.25 mL/min)
Ionization mode: ESI+
Acquisition mode: MRM (see Table 1 for ion transitions)
Capillary voltage: 1 kV
Collision Energy: Optimized cone voltage for each compound: Optimized data management for each compound: MassLynx software
Initial Condition Screening <br>This experiment used a column manager to examine the columns of four different chemical fillers and four different auxiliary solvents, and screened a series of operating conditions to determine the use of UPC 2 for the analysis of opioids. The best starting conditions. The columns used were Waters® UPC 2 BEH, BEH 2-EP, CSHTM fluoro-phenyl and HSS C18 SB. Mobile phase B was methanol with the following additives: none (methanol only), 0.2% formic acid, 0.2% NH4OH or 0.2% formic acid + 20 mM NH4COOH. The gradient of the initial screening experiment started with 5% mobile phase B and increased from 5% to 75% in 4 min. Then return to 5% in 1 min and re-equilibrate the column for 1.4 min under initial conditions. The flow rate for each column was set to ensure that the system back pressure was below the 6000 psi limit, the flow rate for the BEH and 2-EP columns was 1.5 mL/min, and the HSS and PFP CSH fluoro-phenyl column flow rate was 1.0 mL/ Min. Initial conditions screening experiments were performed using several compounds including fentanyl, morphine, oxymorphone, oxycodone, and methadone. These compounds represent a range of polarities that simplify the initial screening process. 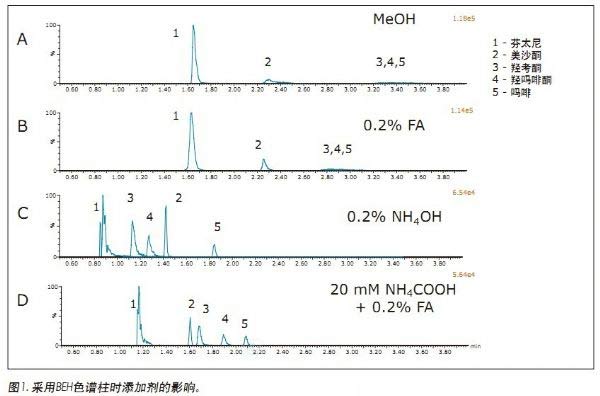
references
1. Janicot J, Caude M, et al. Separation of opium alkaloids by carbon dioxide sub- and supercritical fluid chromatography with packed columns; Application to the quantitative analysis of poppy straw extracts. Journal of Chromatography. 1988; 437: 351-364.
2. Grand-Guillaume Perrenoud A, Boccard J, et al. Analysis of basic compounds by supercritical fluid chromatography: Attempts to improve peak shape and maintain mass spectrometry compatibility. Journal of Chromatography A. 2012;1262(0): 205-213.
3. ChemAxon. Chemicalize.org. Retrieved Dec 15, 2012, from http://
4. Lesellier E. Retention mechanisms in super/subcritical fluid chromatography on packed columns. Journal of Chromatography A. 2009; 1216(10): 1881-1890.