The FDA certification rate for wearable ECG monitoring is nearly 50%. How does the market play in the next step?
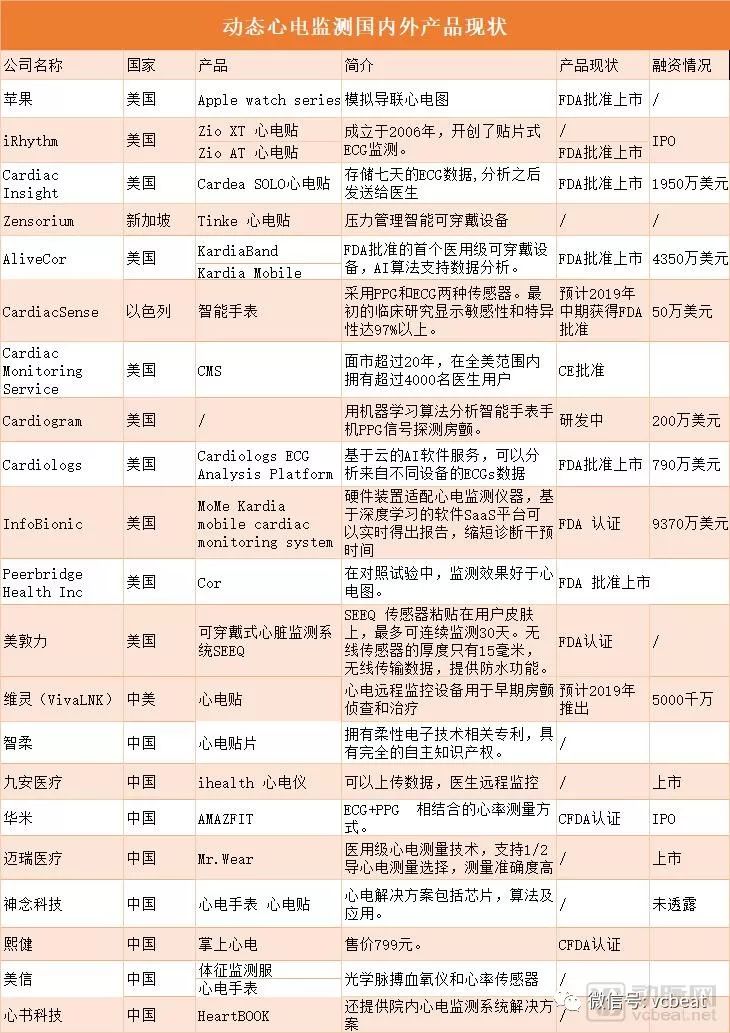
2012 is the first year of consumer wearables, and 2018 is the first year of medical wearables. In fact, this time should be released abroad in 2017. The arterial network statistics found that many of the FDA-approved dynamic ECG monitoring products were approved in 2017. The milestone in 2018 was Apple's release of iWatch with FDA-approved ECG detection capabilities, which allowed the entire dynamic ECG market to boil again. Domestically, Huami's AMAZFIT is the first CFDA-certified medical -grade wearable dynamic ECG recorder. At what stage of development is wearable dynamic ECG monitoring at home and abroad? After getting the authority certification, what are the next trends? The arterial network took stock of the main players in the wearable dynamic ECG monitoring market at home and abroad, and interviewed relevant industry insiders to find two major trends: 1. After breaking through the CFDA/FDA certification mark, the next step will be to connect doctors and diagnoses. 2, one mountain can accommodate two tigers, bracelets and ECG stickers will be further integrated. How will the dynamic ECG monitoring market break through the stock market and incremental market? In the first year of medical wearable devices, wearable products began to play the role of product screening and auxiliary diagnosis. For the four vital signs: body temperature, heart rate, blood pressure, respiratory rate have product breakthroughs, in addition to blood sugar, ECG, EEG, blood oxygen and other signs are also a battleground. Especially in heart rate monitoring, wearable devices can monitor atrial fibrillation and other hard-to-find arrhythmias. At present, Holter ECG wearable devices can more easily solve the medical needs of patients with arrhythmia. Similarly, some symptoms such as palpitations, dizziness, shortness of breath, or unexplained fainting may indicate the symptoms of arrhythmia. Dynamic ECG monitoring equipment. At the same time, there are some people who are trying to advance arrhythmia monitoring to asymptomatic patients, although at present it is controversial how to define high-risk groups of atrial fibrillation and the general population. It is important to use data to demonstrate the benefits of ordinary people wearing dynamic ECG monitoring. Medical grade ECG monitoring products want to meet the needs of these two types of people. First, they must have accuracy and become an auxiliary diagnostic tool, but they must also be as smart, lightweight and friendly as a consumer product. Atrial fibrillation may cause a stroke, and data from the US Centers for Disease Control and Prevention show that about 15-20% of ischemic strokes are caused by atrial fibrillation. Doctors with atrial fibrillation in high-risk strokes usually use anticoagulant drugs as a preventive measure. Low-risk AF patients can reduce the risk of stroke by changing lifestyles. But the premise is that they have to know that they are at risk. The researchers said that most patients with high-risk or low-risk were not prevented and screened early. At present, the entire US dynamic heart rate monitoring is a market of 1.4 billion US dollars per year. This market is still growing, considering that the launch of Apple Watch with ECG products will bring more traffic. The prevalence of atrial fibrillation is also closely related to diseases such as coronary heart disease, hypertension and heart failure. In our country, the number of sudden deaths due to heart disease each year exceeds 500,000. According to the "China Health and Family Planning Statistical Yearbook" issued by the National Health and Family Planning Commission, cardiovascular disease has become the leading cause of death among residents in China, higher than cancer and other diseases. The current dynamic ECG monitoring mainly adopts two kinds of signal collection methods: ECG and PPG. The ECG is detected by bioelectricity. After capturing the bioelectric signal and then digitizing it, it can be converted into digital signal processing, and then the output can be accurately output. Detailed heart health information. PPG refers to photoplethysmography (PhotoPlethysmoGraphy), or PPG for short, to monitor heart rate. The principle is simple: the blood is red, reflecting red light and absorbing green light. PPG, through the detection of the amount of blood circulating at the wrist at a specific time, to obtain heart rate information. Another classification method is the difference in lead. A standard ECG requires 12 leads, 12-lead automatic ECG analysis system, capable of 12-lead, 6-lead, 3+1 lead, 3-lead, long-term rhythm lead record; recordable In a conventional ECG, if an abnormal heart rhythm is found, the 1-minute waveform recording and extension recording of the rhythm lead can be automatically completed. Most of the dynamic ECG monitoring products are analog leads. Take the ECG of apple watch as an example, there is only one lead. Secondly, this lead is not a standard lead collected by standard methods, but after other methods are used to collect the signal, the software program calculates the graph that the doctor is used to see, which is the analog lead. Recently, AliveCor announced the company's next direction: the introduction of a six-lead ECG compatible with smartphones. Perhaps one day wearable devices will also become medical monitoring products. After breaking through the CFDA/FDA certification mark, the next step will be to assist diagnosis In the consumer-grade wearable device bubble of 2012, healthy wearables fell into the predicament of homogenization and the collection of data. But now the entire market for wearable dynamic ECG monitoring equipment has begun to have new thresholds, with new rules of the game. There are traditional medical device companies as well as technology giants and startups are standing on the new starting line. After Apple released the Series 4 Apple Watch with ECG monitoring, Apple can be said to be the main player in this field. Apple's Series 4 Apple Watch ECG monitoring feature is FDA-approved. Apple Watch will issue a warning after monitoring the heart rate market. The user can then place the finger on the crown to collect the ECG signal. After 30 seconds, the ECG APP can be used to see the analog single-lead ECG. But there is no need to regard Apple as the number one enemy of wearable dynamic ECG monitoring, because Apple can promote atrial fibrillation monitoring to more consumer groups, who may not have a high risk of atrial fibrillation. From the short-term perspective, a large number of Apple users can provide a large amount of data to prove the benefits of dynamic ECG monitoring. Apple has conducted a study that has more than 400,000 participants in the Heart Study, the largest screening study of atrial fibrillation to date, and one of the largest cardiovascular trials to date. A portion of the data from the Apple Heart Study was submitted to the FDA to support the approval of heart rate anomaly monitoring products. Transforaminal Endoscopy Instruments We're professional Transforaminal Endoscopy Instruments
manufacturers and suppliers in China, specialized in providing high
quality medical instruments with reasonable price. We warmly welcome you
to buy or wholesale bulk Transforaminal Endoscopy Instruments for sale here and get quotation from our factory. Transforaminal Endoscopic,Transforaminal Locating Ruler,Transforaminal Biting Forceps,Transforaminal Endoscopy Instruments Tonglu WANHE Medical Instrument Co., Ltd , https://www.vanhurhealth.com