Q Exactive Focus High Resolution Liquidopharmaceutical Impurity Analysis Solution
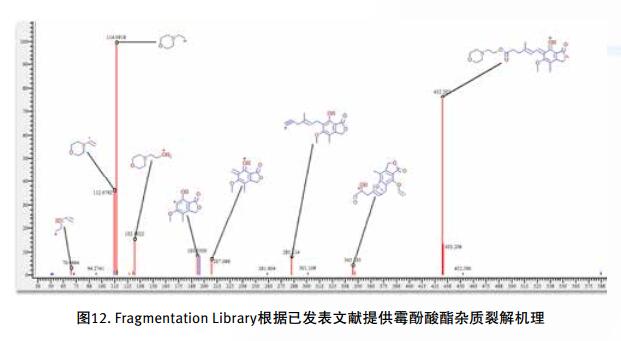
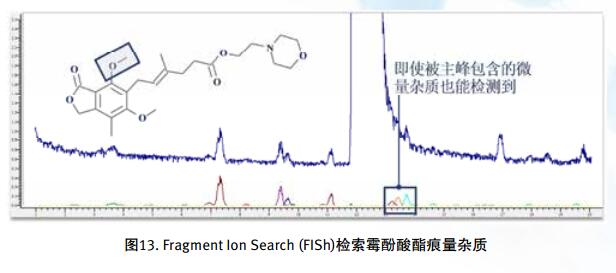
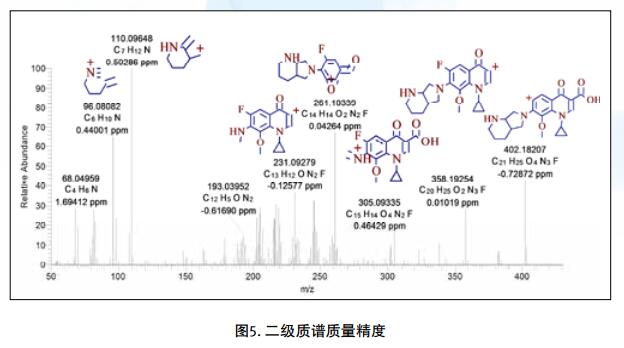
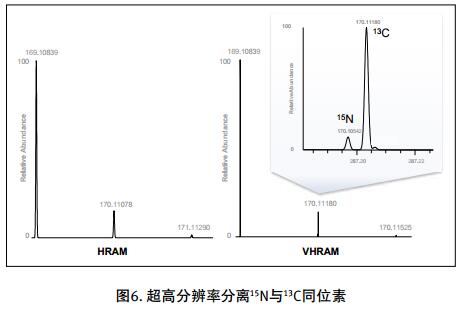
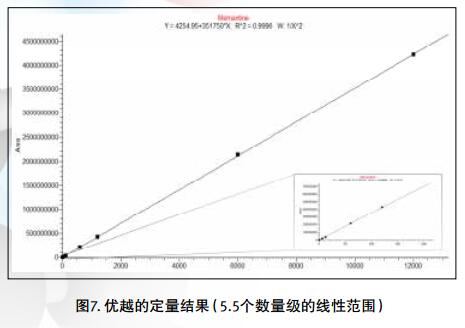
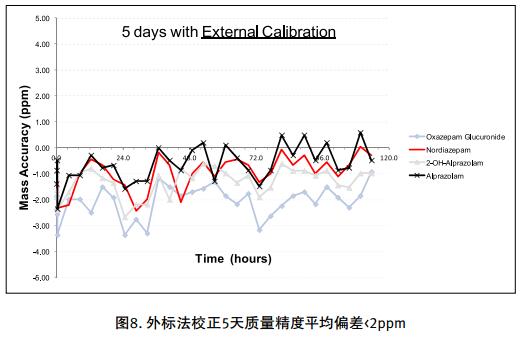
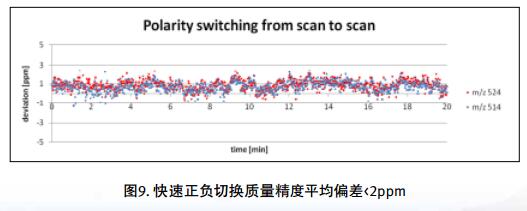
Drug impurities have become an important concern of drug regulatory agencies at home and abroad because of their potential impact on drug quality, safety and efficacy. With the expansion of the export scale of China's pharmaceutical products, understanding the control requirements of drug impurities in foreign regulatory markets and strengthening the analysis and control of drug impurities have become a common concern of domestic pharmaceutical manufacturers. Any substance that affects the purity of a drug is collectively referred to as an impurity. The International Drug Coordination Council (ICH) is defined by the International Harmonization Council (ICH) as an ingredient present in a drug that is inconsistent with the drug. Impurities in the drug will reduce the efficacy, affect the stability of the drug, and some may even be harmful to human health or produce other toxic side effects. Therefore, the detection of related substances, control of purity is very important to ensure the safety and effectiveness of medication, to ensure the quality of the drug. Impurity spectrum analysis refers to the distribution of known and unknown impurities present in the study drug, and analyzes the source and destination of impurities in the drug. Through the study of the impurity spectrum, the safety of the drug can be comprehensively evaluated. For the drug production stage, the impurity spectrum study can establish a complete and reliable impurity analysis method in the process, monitor the change of impurities in the key steps of the process, verify the impurity analysis method and transfer to QA/QC, for the drug development stage, it needs to The impurities in the process of process development are identified and characterized and the source of the impurities is further confirmed. Based on the analysis results, the R&D personnel can evaluate the safety of the drug and the consistency with the original drug, and further optimize the process according to the source of impurities to reduce or eliminate the generation of impurities. . The organic impurities mainly originate from the starting materials and the impurities contained in the raw materials, the synthetic intermediates and side reaction products brought in the production process, the degradation products produced by the finished products during the storage process, and the impurities or auxiliary materials brought in by the auxiliary materials are degraded by interaction. Impurities, and impurities produced by the interaction of the drug and the package. With the continuous development of high-resolution mass spectrometers in recent years, it has become a common equipment in the laboratory. With ultra-high resolution, high-resolution mass spectrometry can accurately measure the exact mass-to-charge ratio of impurities, and then calculate the elemental composition of impurities, and distinguish between impurities with close masses and co-eluting components in complex backgrounds, with complex matrices Analytical ability of trace impurities. The high-resolution full-scan method does not miss the information of unknown impurities. It is a powerful tool for researching target or non-target drug impurities, and it is used more and more in the field of drug quality research. Impurity analysis workflow The impurities in the drug are often very small. In the presence of a large amount of the parent drug, the detection of trace impurities is a major challenge to mass spectrometry. After collecting the mass spectrometry information of the impurities, the structure of these process impurities or degradation products is effective. The analysis is another big challenge. Thermo Fisher's electrostatic field orbitrap mass spectrometry effectively solves the above impurity research problems. Its high sensitivity and wide dynamic range enable the collection of effective mass spectral information of trace impurities. Ultra-high resolution can effectively distinguish impurities and interfering substances. It is possible to distinguish all co-eluting components by high resolution without spending a lot of time on optimization of chromatographic conditions. The CD High Energy Collision Pool provides rich debris and combines the high quality accuracy of the primary and secondary to obtain effective elemental composition and structural information. Combining the efficient separation of chromatography with professional structural analysis software, Thermo Fisher has established a solution specifically for impurity analysis. Orbitrap electrostatic field orbitrap high resolution mass spectrometry technology introduction â— Orbitrap's high performance and reliability have been proven by the market. Its applications range from drug development, food safety, environmental monitoring, and judicial testing, including 1. Drug discovery and development, metabolite identification, and impurity research. 2. Food safety chemical residue screening; 3. Environmentally hazardous substance monitoring; 4. Sports competition stimulant confirmation test; 5. Forensic drug toxicant detection and other fields Q Exactive Focus quadrupole electrostatic field orbitrap benchtop high resolution mass spectrometry â— High-performance quadrupole with accurate mother ion selectivity and efficient ion transport, effectively reducing noise â— Resolutions up to 70,000 FWHM and < 1 ppm mass accuracy for reliable identification â— Multiple detection and advanced signal processing Technology is better compatible with UHPLC, ensuring data acquisition speeds to increase throughput â— Fast and efficient positive and negative switching in MS and MS/MS modes ensures more compounds are detected in a single run High quality accuracy for accurate impurity identification results The Q Exactive Focus scan speed is perfectly compatible with UHPLC. In the detection of carbaryl, both the low concentration data points and the high concentration data points Q Exactive Focus achieve an accurate molecular weight of less than 1 ppm. High-quality accuracy runs through the entire sampling process, and the data at each sample point is trusted, without the need for spectral averaging, to produce accurate elemental composition. After the element composition of the parent ion is generated, in order to further analyze the impurity structure, it is also necessary to calculate the elemental composition of the fragment ion, and the mass accuracy of the second-order mass spectrum directly affects the accuracy of the composition of the fragment element. Q Exactive Focus maintains high mass accuracy for secondary debris, ensuring elemental composition and resolution of the cracking mechanism. Ultra high resolution unlocking impurity isotope information High-resolution, high-accuracy ( HRAM ) primary and secondary mass spectrometry data is a powerful tool for unknown impurity analysis, but this is not the only basis for structural analysis. Q Exactive Focus has a higher resolution than conventional high resolution ( 70,000 FWHM ), ultra-high resolution can be used to further unlock the isotope information. In the following figure, the 15 N and 13 C isotopes can be separated, thereby judging that the compound contains elements C and N , further through both isotopic abundances. ratio, can be judged that the compound C and the elemental ratio of N to obtain a more accurate result of identifying the only impurity. High sensitivity for superior impurity quantification In the impurity quantification study, the Q Exactive Focus system has the sensitivity and quantification capabilities comparable to the triple quadrupole mass spectrometer, allowing for the good detection and identification of low-level genotoxic impurities required by regulations. Unlike the commonly used selective ion monitoring (SIM) and selective reaction monitoring (SRM) scanning methods for quadrupole mass spectrometry, Q Exactive Focus does not require the use of standards to build libraries and methods, as long as the molecular formula information of the compound can be used to quantify the signal. Extraction. Its ultra-high resolution distinguishes background matrix interferences and achieves sensitivity and quantification capabilities comparable to triple quadrupole mass spectrometry. It can detect and identify low-level genotoxic impurities required by regulations. A full-scan quantitative method can be used to record information on all known and unknown impurities in the sample, facilitating retrospective analysis of the data. Superior stability of the mass axis The long-term quality axis stability test results show that Q Exactive Focus is highly resistant to the laboratory environment and can maintain long-term stable mass accuracy under temperature fluctuations using only external calibration. The ionization efficiency of impurities in drugs between different ionizing polarities may vary greatly. The fast switching between positive and negative polarity between scans in MS and MS/MS modes ensures that the Q Exactive Focus system maximizes the detection and identification of impurities in a single chromatographic run. data analysis There are many kinds of impurities and a wide range of sources. Even compounds with the same element composition may have completely different structures. Therefore, professional analysis software is needed to analyze the collected data, and to effectively extract and analyze possible impurities. The structure is judged. Professional impurity analysis software Compound Discoverer is an intelligent small molecule compound discovery and identification software. Researchers use the software to easily implement the following features: â— Grouping and batch processing of samples under different conditions according to preset classification; Mass Frontier's professional structural analysis software provides sophisticated features for the management, evaluation and analysis of mass spectrograms. By calling rich fragmentation data, the structure of the parent drug fragments can be accurately guessed and attributed. By comparing the impurities with the parent drug, the impurity change sites can be further speculated, and finally a comprehensive impurity identification can be obtained. result. Using Mass Frontier software, researchers can easily get the following information from the collected data: â— Cracking mechanism of the compound Because the structure of the impurity and the structure of the parent drug are often similar or have a relationship, understanding the cleavage law of the parent drug can help determine the fragment structure of the impurity and further infer the structure of the impurity. Previous methods relied solely on the individual experience of the researcher, or purely theoretical calculations, which limited the reproducibility and accuracy of data analysis. The Fragmentation Library fragmentation mechanism library contained in Mass Frontier is collected from all existing publications on mass spectrometry and covers almost all published literature. Each mechanism, along with its chemical structure, is manually and automatically checked and then stored in the library together with additional information such as title, author, and information source. The information collected by the spectral library, combined with general ionization, fragmentation and recombination rules, provides a strong guarantee for the prediction of the fragmentation mechanism. Mass Frontier also allows users to create spectral libraries based on customer-defined fragmentation mechanisms and to learn and apply these mechanisms to intelligently predict fragmented molecules. â— Seizure of related substances Some impurity components may be neglected by researchers because the signal response is too low, or the chromatographic peak formed is not sharp enough, but the low content of toxic impurities seriously affects drug safety. Leak detection will not only greatly affect the safety of medication. It will also affect the new drug declaration indirectly. Mass Frontier's Fragment Ion Search (FISh) allows rapid screening of structurally similar compounds by filtering a set of cleavage fragments, either by theoretical cleavage prediction or by experimental MSn mass spectral tree, by looking for impurity-to-parent fragmentation , accurately find all the relevant impurities. Coverall Products,Durability Surgical Masks,Disposible Face Masks,Safety Protective Coveralls Zhende Medical Co.,Ltd , https://www.zhendemedicals.com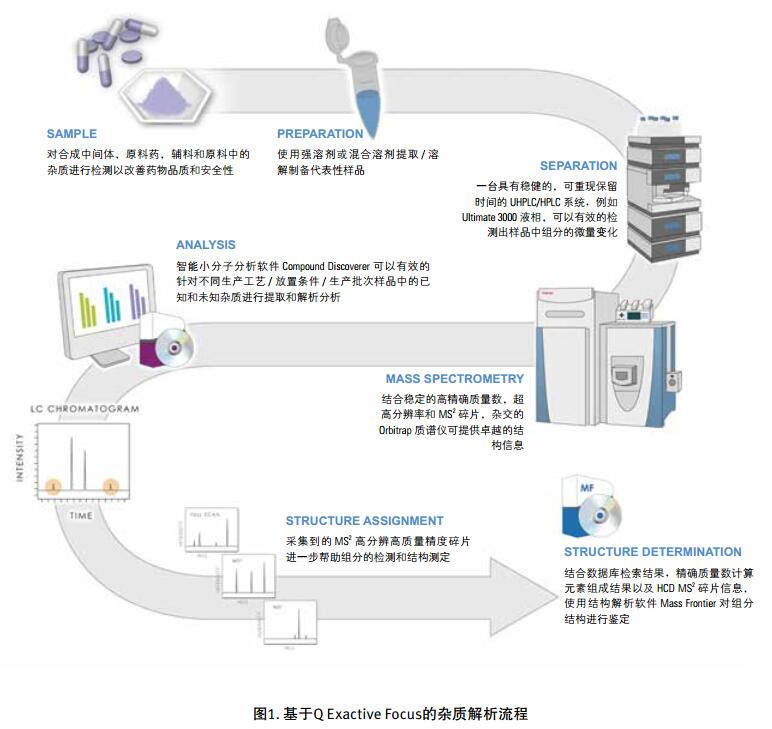
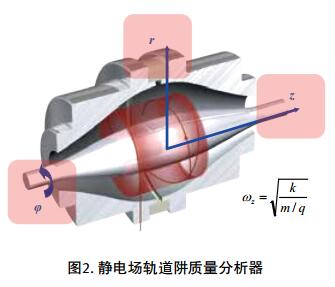 â— Electrostatic field orbitrap mass spectrometry is a new high-resolution mass spectrometer in recent years. It is a new type of mass spectrometer invented by Russian scientist Makarov in 2000. Its mass analyzer is shaped like a scorpion, and it is shaped like a scorpion. The inner electrode of the center is composed of two outer and outer half-electrodes. When the instrument is working, a DC high voltage is gradually applied to the center electrode to generate an electrostatic field of a special geometry in the Orbitrap. When the ions enter the Orbitrap chamber, they are subjected to the gravitational force of the central electric field, and then begin to orbit around the center electrode. At the same time, the ions are subjected to centrifugal force in the vertical direction and thrust in the horizontal direction, and the electrodes in the center are oscillated horizontally and vertically. The outer electrode limits the operating orbital range of the ion and simultaneously detects the induced potential generated by the ion oscillation. The relationship between the frequency of the horizontal oscillation and the mass-to-charge ratio (m/z) of the molecular ion can be described by the mathematical formula on the right. The signal output from each of the external electrodes of Orbitrap is amplified by a differential amplifier and converted from a fast Fourier transform to a spectrum, which in turn is converted to a mass spectrum.
â— Electrostatic field orbitrap mass spectrometry is a new high-resolution mass spectrometer in recent years. It is a new type of mass spectrometer invented by Russian scientist Makarov in 2000. Its mass analyzer is shaped like a scorpion, and it is shaped like a scorpion. The inner electrode of the center is composed of two outer and outer half-electrodes. When the instrument is working, a DC high voltage is gradually applied to the center electrode to generate an electrostatic field of a special geometry in the Orbitrap. When the ions enter the Orbitrap chamber, they are subjected to the gravitational force of the central electric field, and then begin to orbit around the center electrode. At the same time, the ions are subjected to centrifugal force in the vertical direction and thrust in the horizontal direction, and the electrodes in the center are oscillated horizontally and vertically. The outer electrode limits the operating orbital range of the ion and simultaneously detects the induced potential generated by the ion oscillation. The relationship between the frequency of the horizontal oscillation and the mass-to-charge ratio (m/z) of the molecular ion can be described by the mathematical formula on the right. The signal output from each of the external electrodes of Orbitrap is amplified by a differential amplifier and converted from a fast Fourier transform to a spectrum, which in turn is converted to a mass spectrum.  Q Exactive Focus is a benchtop high-resolution mass spectrometer based on Orbitrap technology that combines high-performance quadrupole precursor ion selectivity with high-resolution accurate mass (HR/AM) Orbitrap detection technology to deliver superior performance and versatility . The Q Exactive Focus mass spectrometer enables high-precision target impurity screening or non-target impurity identification, and high-quality data provides more reliable qualitative and quantitative detection of impurities.
Q Exactive Focus is a benchtop high-resolution mass spectrometer based on Orbitrap technology that combines high-performance quadrupole precursor ion selectivity with high-resolution accurate mass (HR/AM) Orbitrap detection technology to deliver superior performance and versatility . The Q Exactive Focus mass spectrometer enables high-precision target impurity screening or non-target impurity identification, and high-quality data provides more reliable qualitative and quantitative detection of impurities. 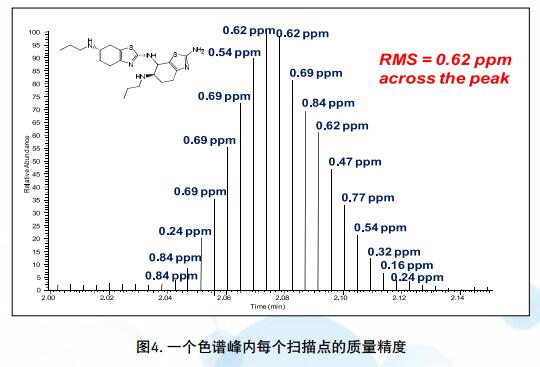
â— Set a custom data processing flow according to research needs;
â— Use a variety of advanced processing technologies: multiple mass loss filtering (MMDF), isotope filtration (IPF), fragment ion retrieval (FISh Scoring) and other functions to extract and analyze expected and unknown related substances;
â— Visually obtain sample difference comparison results under different conditions in the results browsing.