Pain new drugs have achieved 3 positive developments and are expected to solve drug abuse
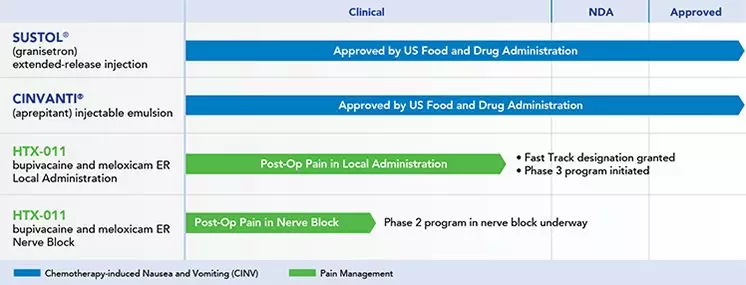
Pain new drugs have achieved 3 positive developments and are expected to solve drug abuse March 20, 2018 Source: WuXi PharmaTech Today, Heron Therapeutics announced positive results in its Phase 3 clinical trial of the new drug HTX-011 in patients with bunion resection (Study 301/EPOCH1) and hernia repair (Study 302/EPOCH2). HTX-011 reached all major and critical secondary endpoints in both Phase 3 studies, demonstrating a significant reduction in pain and opioid use within 72 hours of surgery. Every day, more than 115 Americans die from excessive use of opioids. The abuse and addiction of opioids has become a national crisis that seriously affects public health and socio-economic well-being, which is inextricably linked to the use of addictive opioid painkillers. HTX-011 uses Heron's proprietary Biochronomer® drug delivery technology, a long-acting sustained-release formulation consisting of a fixed-dose local anesthetic bupivacaine and the anti-inflammatory drug meloxicam. For the prevention of postoperative pain. By directly delivering anesthetic and topical anti-inflammatory agents directly to the site of tissue damage, HTX-011 is effective in relieving pain while reducing the need for systemic administration of painkillers (such as opioids) to avoid possible Side effects such as abuse and addiction. In three different surgical models (bunion resection, hernia repair, and abdominal wallplasty), HTX-011 showed more significant pain relief than placebo or bupivacaine alone. The drug was granted a fast track qualification by the US FDA in the fourth quarter of last year, and Heron is expected to submit a new drug application (NDA) to the FDA in the second half of this year. The primary and secondary endpoints of the two Phase 3 studies were the same. The primary end point was the intensity of pain measured using the area under the curve (AUC 0-72) from 0 to 72 hours postoperatively compared to placebo. Key secondary endpoints included: AUC 0-72 pain intensity compared to bupivacaine solution; opioid use compared to placebo 72 hours after surgery; postoperative versus bupivacaine solution The proportion of patients with opioids; and the total opioid use compared with bupivacaine 72 hours after surgery. The results showed that EPOCH1 (bunion resection) reached all major and critical secondary endpoints: Pain intensity (AUC 0-72) was reduced by 27% (p < 0.0001) in the HTX-011 group compared with placebo. The pain in these patients was reduced by 18% compared with bupivacaine solution (p=0.0002). At 72 hours after surgery, the HTX-011 group had 37% less opioids (p<0.0001) than the placebo group and 25% less than the bupivacaine solution group (p=0.0022). At 72 hours after surgery, 29% of patients in the HTX-011 group did not require opioids, compared with 2% in the placebo group (p<0.0001) and 11% in the bupivacaine solution group (p =0.0001). These results indicate a significant reduction in severe pain in the HTX-011 group, which is 36% lower than the placebo group (p<0.0001) and 29% lower than the bupivacaine solution group (p<0.0001). EPOCH2 (Helium Repair) also reached all major and critical secondary endpoints: Pain intensity (AUC 0-72) was reduced by 23% in the HTX-011 group compared with placebo (p=0.0004). These patients had a 21% reduction in pain compared to bupivacaine solution (p < 0.0001). At 72 hours after surgery, the HTX-011 group had 38% less opioids (p=0.0001) than the placebo group and 25% less than the bupivacaine solution group (p=0.0240). At 72 hours after surgery, 51% of patients in the HTX-011 group did not require opioids, compared with 22% in the placebo group (p<0.0001) and 40% in the bupivacaine solution group (p =0.0486). These results indicate a significant reduction in severe pain in the HTX-011 group, which is 40% lower than in the placebo group (p<0.0001) and 19% lower in the bupivacaine solution group (p=0.0372). In addition, HTX-011 was well tolerated in both studies and was comparable in safety to placebo and bupivacaine solutions. ▲ Heron Therapeutics clinical pipeline (Source: Heron Therapeutics official website) "Using opioid analgesics to acutely control postoperative pain is directly related to the continuous use of more than 2 million new opioids per year. The number of new cases of opioid use disorders is up to 440,000 per year, making postoperative opioid use a US An important cause of opioid abuse. In addition, more than 1 billion opioids are used every year after surgery to control pain, which poses a huge potential for the diversion of these drugs, and there is an urgent need for non-opioid substitutes. Dr. Eugene R. Viscusi, professor of anesthesiology and director of anesthesiology pain medicine at Sidney Kimmel School of Medicine at Thomas Jefferson University, said: "The results of Phase 3 clinical trials of HTX-011 suggest that it may be a potential for a wide range of surgical procedures. Non-opioid drugs for multimodal pain management." “My family has experienced an indescribable grief. Our son, Taylor, died of overdose of heroin. If he did not start using opioids after a routine elbow surgery, this tragedy could have been avoided. Doctors, patients and families need A new, more effective pain management program to address post-operative pain problems to reduce the number of people who use harmful opioids and block it before addiction," said Trace United founder Travis Bornstein. "Based on today's findings, HTX-011 is the only topical drug that can significantly relieve pain and reduce opioid use in a phase 3 study compared to placebo and standard care regimen bupivacaine solution. Anesthetic," Dr. Barry D. Quart, CEO of Heron, said: "We look forward to submitting a new drug for HTX-011 to the US FDA in the second half of 2018. If approved, we believe that HTX-011 can reduce postoperative opioids. The use of drugs has a major impact on the opioid crisis while reducing the pain of patients." Reference materials: [1] Heron Announces Positive Topline Results From Pivotal Phase 3 Clinical Trials of HTX-011 in Bunionectomy and Hernia Repair [2] Heron Announces Official Website [3] National Institute on Drug Abuse 1)High-luminance and white LED, features in long life and good stability. Urine Analyzer,Urine Analyser,At Home Water Test,Water Indicator Paper Changchun LYZ Technology Co., Ltd , https://www.lyzstrips.com
2)Large LCD screen, high luminance, abundant contents display, optional languages: Chinese and English.
3)User-friendly interface.
4)Optional units: international unit, conventional unit and symbol system.
5)Three working mode: one-step/slow/fast testing mode, suitable for different user group.
6)Monitoring the whole test process, auto-character and audible prompt.
7)Be compatible with 8, 10 and 11-parameter test strip.
8)Standard RS232 interface and interface for data communication.
9)Built-in thermal printer.