Surgical Blade/Disposable Surgical Blade with Plastic Handle /Surgical Scalpel
Principle
sandwich method and gold immunochromatography assay.
Main components LH Ovulation Rapid Test Kits,Medical Supplies Ovulation Rapid Test,Ovulation Urine Test Strips,LH Ovulation Luteinizing Hormone Rapid Test Kit Changchun ZYF science and technology CO.,LTD , https://www.zyf-medical.com
Model: ZG-FET007
Specification
A) Material: Carbon steel/Stainless Steel
B)Specifications: 11#, 12#, 13#, 14#, 15#, 15C, 18#, 19#, 20#, 21#, 22#, 23#, 24#, 25#, 36#
C) Sterilization method: Gamma Radiation 25 KGY
D) Inner packing: 1/sealed aluminimum foil bag, 100pcs/box
E) Outer packing: 5000pcs/ctn
Disposable Surgical Blades
Surgical Blades
Material: Carbon Steel, Stainless Steel
Packing: Individual packed in Aluminum foil
Sterilization: Gamma Ray
Gouge Blades Stitch Cutter
Material: Carbon Steel, Stainless Steel
Packing: Individual packed in Aluminum foil
Sterilization: Gamma Ray
Stainless Steel Scalpel Handle/Disposable Scalpels
Material: Carbon Steel, Stainless Steel
Packing: Individual packed in Aluminum foil
Sterilization: Gamma Ray
Disposable Sterile Surgical Blades
Model: ZG-FET007
Specification
A) Material: Carbon steel/Stainless Steel
B)Specifications: 11#, 12#, 13#, 14#, 15#, 15C, 18#, 19#, 20#, 21#, 22#, 23#, 24#, 25#, 36#
C) Sterilization method: Gamma Radiation 25 KGY
D) Inner packing: 1/sealed aluminimum foil bag, 100pcs/box
E) Outer packing: 5000pcs/ctn
Disposable Surgical Blades
Surgical Blades
Material: Carbon Steel, Stainless Steel
Packing: Individual packed in Aluminum foil
Sterilization: Gamma Ray
Gouge Blades Stitch Cutter
Material: Carbon Steel, Stainless Steel
Packing: Individual packed in Aluminum foil
Sterilization: Gamma Ray
Stainless Steel Scalpel Handle/Disposable Scalpels
Material: Carbon Steel, Stainless Steel
Packing: Individual packed in Aluminum foil
Sterilization: Gamma Ray
The test utilizes antibodies including a monoclonal LH-β antibody and goat anti-rat IgG on the
nitrocellulose membrane with colloidal gold marked anti-LH-α monoclonal antibody as an mark
tracer. The reagent is used to detect the LH in urine according to the principle of double antibody
The testing kit is in the form of strip, cassette, and midstream. Basic components: Sample pad,
colloidal gold marked pad, nitrocellulose membrane, absorbent paper and PVC board. Colloidal
gold marked pad coated with LH-α monoclonal antibody, nitrocellulose membrane coated with
LH-β monoclonal antibody,control line coated with goat anti-rat IgG.
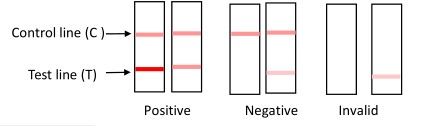
Result Judgment
POSITIVE: Two distinct red lines appear. If the color of testing line is stronger than or equal to the
control line, it demonstrates that the ovulation is about to occur in the next 24 to 48 hours.
NEGATIVE: One red line appears in the control region(C). Or both red line appear with the color of
testing line is weaker than the control line, it demonstrates that the ovulation period does not
arrive yet.
INVALID: No red lines appear or control line fails to appear, indicating that the operator error or
reagent failure. Verify the test procedure and repeat the test with a new testing device.