$5.1 billion! GSK acquires TESARO to expand cancer research and development pipeline
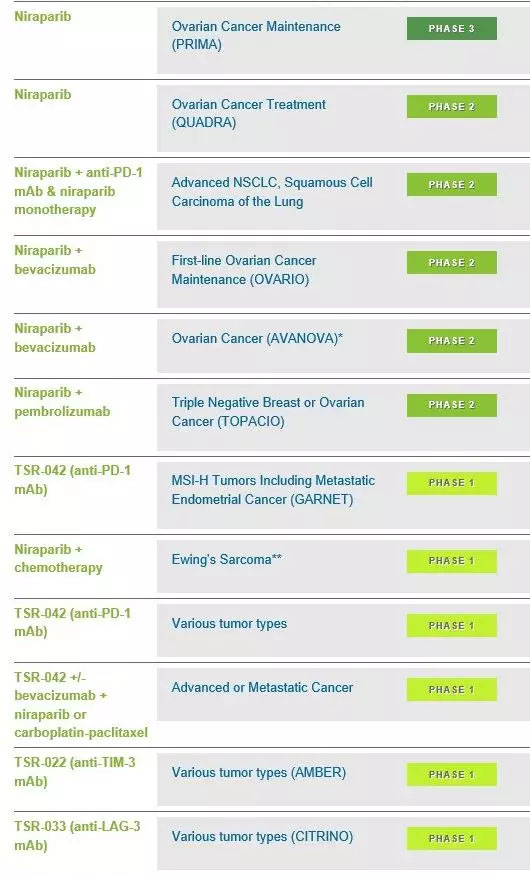
Recently, GlaxoSmithKline announced that the company will acquire TESARO, a Massachusetts-based oncology drug research and development company. The two companies have reached a definitive agreement to acquire $75 per share in cash for a total price of approximately $5.1 billion (40). 100 million pounds), including the net debt of TESARO, the price is 110%. It is understood that TESARO is a biopharmaceutical company in the commercial stage. The main product is Zejula (niraparib), an oral poly ADP ribose polymerase (PARP) inhibitor currently approved for ovarian cancer. PARP inhibitors are altering the treatment of ovarian cancer, particularly in patients with and without germline mutations in the BRCA gene (gBRCA). Zejula is currently approved in the United States and Europe for the treatment of adult patients with recurrent ovarian cancer who respond to platinum-based chemotherapy regardless of BRCA mutations or biomarker status. For the nine months ended September 30, Zejula's 2018 income as a second-line maintenance treatment for ovarian cancer was $166 million. At the same time, clinical trials evaluating Zejula as a monotherapy and combination in the “all participants†patient population are also underway to assess the opportunity to significantly increase first-line maintenance therapy for ovarian cancer. These ongoing trials are evaluating the potential benefits of Zejula for patients with gBRCA mutations and for larger populations without gBRCA mutations and tumors that are HRD-positive and HRD-negative. The first results of a clinical trial codenamed PRIMA are expected to be 2019. Published in the second half of the year. It is worth mentioning that Zejula is developed in China by Ding Ding Medicine. On October 22, 2018, Ding Pharmaceutical announced that Zejula was approved in Hong Kong. The approval was based on its international phase III clinical trial ENGOT-OV16/ NOVA study, initiated by TESARO, is a double-blind, placebo-controlled study enrolling 553 platinum-sensitive recurrent high-grade serous ovarian, fallopian, or primary peritoneal cancer patients with platinum-sensitive Defined as complete or partial remission for more than 6 months after the penultimate platinum-containing chemotherapy, the test results showed that Zejula significantly prolonged PFS regardless of whether the patient had germline BRCA mutations compared with the control group. At the same time, treatment with Zejula reduced the risk of disease progression or death in germline BRCA mutant patients by 73% (hazard ratio (HR) 0.27), and reduced the risk of disease progression or death in patients without germline BRCA mutation by 55%. (HR 0.45), patients with complete or partial remission with platinum-containing chemotherapy can benefit from Zejula's maintenance therapy. GSK believes that PARP inhibitors provide an important therapeutic opportunity for the treatment of multiple cancer types. In addition to ovarian cancer, Zejula is currently being studied for lung cancer, breast cancer and prostate cancer, either as a monotherapy or in combination with other drugs, including TESARO's anti-PD-1 antibody dostarlimab (formerly known as TSR- 042). In addition, TESARO has a series of clinically-researched immunotherapy drugs, which target immune checkpoints including PD-1, TIM-3 (T-cell immunoglobulin and mucin domain-3) and LAG- 3 (lymphocyte activation gene-3). PD-1 can limit T cell-mediated immune responses, TIM-3 acts as a pattern recognition receptor to suppress anti-tumor immune responses, and LAG-3 is a negative regulator of T cell activity. Antibodies against these targets may restore immune anti-cancer function in a variety of tumor types by blocking the interaction of PD-1, TIM-3 and LAG-3 with their respective ligands. TESARO's product development pipeline (Source: TESARO official website) For the acquisition, GSK CEO Emma Walmsley said: "The acquisition of TESARO will accelerate the development of our tumor pipeline and commercial footprint, which will accelerate the company's pharmaceutical business, while bringing new research capabilities. The portfolio will support our goal of achieving long-term sustainable growth and be consistent with our capital allocation priorities." Safe Box With Watch Winder,Storage Safe Box,Jewelry Box,Portable Jewelry Box Hebei Yingbo Safe Boxes Co.,Ltd , https://www.ybsafebox.com